Plastically bendable pregabalin multi-component systems with improved tabletability and compressibility†
Abstract
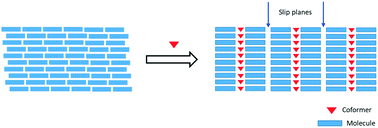
Pregabalin is an anti-convulsant blockbuster chiral drug with the trade name Lyrica in its S-form. Anhydrous pregabalin (SPG) is brittle in nature and herein, we report two novel plastically bendable SPG salts with pharmaceutically acceptable coformers oxalic acid and salicylic acid. These mechanically flexible salts show improved solubility, tabletability and compressibility compared to the marketed anhydrous form.


 Please wait while we load your content...
Please wait while we load your content...