Low temperature growth of (AlGa)2O3 films by oxygen radical assisted pulsed laser deposition
Fabi
Zhang
 ab,
Congyu
Hu
a,
Makoto
Arita
c,
Katsuhiko
Saito
a,
Tooru
Tanaka
a and
Qixin
Guo
ab,
Congyu
Hu
a,
Makoto
Arita
c,
Katsuhiko
Saito
a,
Tooru
Tanaka
a and
Qixin
Guo
 *a
*a
aDepartment of Electrical and Electronic Engineering, Synchrotron Light Application Center, Saga University, Saga 840-8502, Japan. E-mail: guoq@cc.saga-u.ac.jp
bGuangxi Key Laboratory of Precision Navigation Technology and Application, Guilin University of Electronic Technology, Guilin 541004, China
cDepartment of Materials Science and Engineering, Faculty of Engineering, Kyushu University, 744 Motooka, Fukuoka 819-0395, Japan
First published on 12th November 2019
Abstract
Low temperature growth of β-(AlGa)2O3 films has been realized by oxygen radical assisted pulsed laser deposition. The prepared films show a good (−201) orientation perpendicular to (0001) sapphire substrates even at a deposition temperature as low as 200 °C. The influences of the substrate temperature on the structural and optical properties of the films have been systematically investigated. All the films grown at substrate temperatures from 100 to 500 °C exhibit a high transmittance of over 90% in the ultraviolet and visible range. Abrupt bandgap value variation has been observed for films deposited at substrate temperatures higher than 100 °C, which agrees with the amorphous to crystalline transition temperature evidenced by X-ray diffraction. The speed of the film thickness decrease with substrate temperature is much slower for (AlGa)2O3 films grown with oxygen radical assistance, indicating the suppression of the evaporation of volatile species with the help of oxygen radical species. The low temperature growth of β-(AlGa)2O3 films could be compatible with the established lithography of semiconductor microfabrication processes.
1. Introduction
(AlGa)2O3, which has a tunable bandgap between 4.9 (Ga2O3) and 8.7 eV (α-Al2O3) and high stability in air at elevated temperatures with respect to other nitride and oxide systems, has recently emerged as a promising material for optoelectronic applications.1 Okumura et al. have demonstrated the assembly of lateral field-effect transistors using Sn-doped β-(AlGa)2O3.2 Feng et al. have found that (AlGa)2O3 can be a good candidate for solar-blind photodetectors.3 A modulation-doped two dimensional electron gas at a β-(Al0.2Ga0.8)2O3/Ga2O3 heterojunction has been realized by Krishnamoorthy et al.4The above mentioned (AlGa)2O3-based solid-state devices are almost based on material structures created by thin film deposition. Thus, the deposition technology can be regarded as one of the keys to the fabrication of (AlGa)2O3-based devices. Various growth techniques have been explored to deposit (AlGa)2O3 films, including sputtering,5 molecular beam epitaxy,6,7 mist chemical vapor deposition,8 metal–organic chemical vapor deposition9 and pulsed laser deposition (PLD).10–12 The reported growth temperature for the crystalline (AlGa)2O3 film was varied from 350 to 800 °C, depending on the deposition technology mentioned above. However, in order to be compatible with the established lithography of semiconductor microfabrication processes, a lower growth temperature is preferred.13,14
Among various deposition methods, PLD is a good candidate technology for low temperature growth of (AlGa)2O3 films because the ablated species generated in the PLD process have relatively high kinetic energies. In another aspect, the oxygen radical has been found to be an effective assistant species for decreasing the growth temperature of thin films. Thus, the combination of PLD and oxygen radical assistance should be a very effective method for low temperature film growth. Shao et al.15 found that oxygen radicals are effective in realizing low temperature growth of nanocrystalline ZnO thin films by PLD. Room-temperature deposition of Al-doped ZnO films by oxygen radical-assisted PLD has been reported by Matsubara et al.16 We have found that β-Ga2O3 thin films with a (−201) orientation can be fabricated at a substrate temperature as low as 200 °C by using plasma-assisted PLD. However, low temperature growth of (AlGa)2O3 films has not been reported till now, to the best of our knowledge.
In this work, we report the growth of (AlGa)2O3 using oxygen radical-assisted PLD. The substrate temperature influence on the structural and optical properties of (AlGa)2O3 has been discussed in detail.
2. Experimental
(AlGa)2O3 films were deposited by the PLD method on (0001) sapphire substrates. Prior to deposition, the substrates were cleaned using an (a) ultrasonic methanol bath, followed by an ultrasonic acetone bath, (b) with a mixture of phosphoric acid and sulfuric acid, and finally, with (c) deionized water. A 99.99% pure (AlGa)2O3 ceramic with an Al2O3 weight content of 20 wt% was set as a target. The (AlGa)2O3 films were deposited using a laser pulse energy of 225 mJ, a repetition rate of 2 Hz, and an oxygen pressure of 0.1 Pa, and the distance between the substrate and the target is 5 cm. Oxygen radicals were generated by passing high purity oxygen gas (99.999%) through an oxygen plasma cell with an RF power set at 300 W. Five samples of the (AlGa)2O3 films were fabricated at substrate temperatures of 100, 200, 300, 400, and 500 °C. The deposition time was 2 hours for all the samples.The thickness of the films was determined using a step profile analyzer. X-ray diffraction (XRD) was carried out on a PANalytical MRD Pro system in order to investigate the crystal structure of the samples. The optical transmission spectra were recorded using a UV-visible spectrometer. X-ray photoelectron spectroscopy (XPS) measurements were performed using an Al Kα X-ray source. The position of the C 1s peak was used as a standard reference. The surface morphologies were studied by atomic force microscopy (AFM) under ambient conditions.
3. Results and discussion
Fig. 1 shows the AFM images of the (AlGa)2O3 films grown at substrate temperatures from 100 to 500 °C. The surface morphologies of the films change with the increase of substrate temperature. Smooth surfaces are obtained for the films deposited at substrate temperatures lower than 300 °C while the surfaces of the films deposited at substrate temperatures of 400 and 500 °C are composed of elongated islands.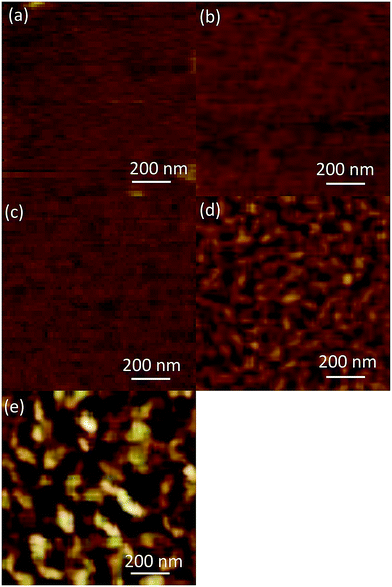 | ||
| Fig. 1 AFM images of (AlGa)2O3 films grown at different substrate temperatures of (a) 100, (b) 200, (c) 300, (d) 400 and (e) 500 °C, respectively. | ||
Fig. 2 shows the thickness of the (AlGa)2O3 films grown at different substrate temperatures. The film thickness shows a decreasing tendency with the increase of substrate temperature. The decrease of film thickness with increasing substrate temperature is often related to the morphologies of the films. The morphology of the film changes with the homologous temperature Ts/Tm (Ts: substrate temperature, Tm: melting temperature of the film material), according to the empirical structure zone model.17 Based on this model, the film becomes dense which results in the decrease of the film thickness with the increase of the substrate temperature.18–21 However, this model seems to be not the main factor because the films deposited at low temperatures show smooth surfaces in our case. The decrease of film thickness with increasing substrate temperature in this work is attributed to the re-evaporation of the adsorbed species on the surface of the substrate. The decrease of film thickness with increasing substrate temperature has also been observed on Ga2O3 films as well as on (AlGa)2O3 films grown by PLD without oxygen radical assistance in our previous work.22,23 However, the speed of film thickness variation ((T300–T500)/T300, where T300 and T500 are the thickness values of films deposited at 300 and 500 °C, respectively) in this work (5.3%) is much slower than that of (AlGa)2O3 films grown without oxygen radical assistance (25%).23 The relatively smaller variation of the growth rate with substrate temperature in this work indicates the suppression of the evaporation of volatile Ga2O species with the help of oxygen radical species.
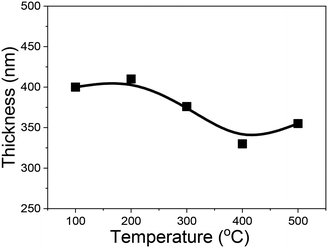 | ||
| Fig. 2 Temperature dependence of film thickness of (AlGa)2O3 films deposited by oxygen radical-assisted PLD. | ||
In order to investigate the stoichiometry of the (AlGa)2O3 films, XPS measurements were carried out. Fig. 3(a) shows the stacked XPS survey scans of the (AlGa)2O3 films deposited at different substrate temperatures. From the spectra, elements Ga, O, C and Al have been observed. No other elements can be found from the survey scans, indicating that high purity (AlGa)2O3 films are obtained. The Ga 2p core level spectra of the (AlGa)2O3 films deposited at different substrate temperatures are shown in Fig. 3(b). In all cases, the binding energy of Ga 2p remains at 1117.7 eV and has been attributed to Ga–O bonding.24 The Al 2p core level spectra of the (AlGa)2O3 films deposited at different substrate temperatures are shown in Fig. 3(c). The Al 2p peaks for the films deposited from 200 to 500 °C are all located at 74.1 eV. The Al 2p peak for the film deposited at 100 °C is located at about 73.9 eV. These values are smaller than that of the pure Al2O3 film (74.5 eV),25 indicating the formation of Al–O–Ga bonds. Hu et al.26 have found that the Al 2p peak shifts to a higher binding energy with increasing Al composition. We have investigated the XPS spectra of the (AlGa)2O3 films with different Al contents and also found that the Al 2p peak shifts towards a higher binding energy with the increase of Al content.25 The Al content (Al/(Ga + Al)) and the oxygen content were estimated from the XPS core level peak area after applying an atomic sensitivity factor, as shown in Fig. 3(d). The Al contents in the films deposited at substrate temperatures from 200 to 500 °C are all about 0.126 while the Al content in the film deposited at 100 °C is 0.14, which agrees well with the peak shift of Al 2p observed in Fig. 3(c). It is noticeable that the oxygen contents in the films deposited at substrate temperatures of 100, 200, 300, 400 and 500 °C are about 0.60, 0.58, 0.58, 0.55 and 0.56, respectively, as shown in Fig. 3(d), verifying the formation of the (AlGa)2O3 films.
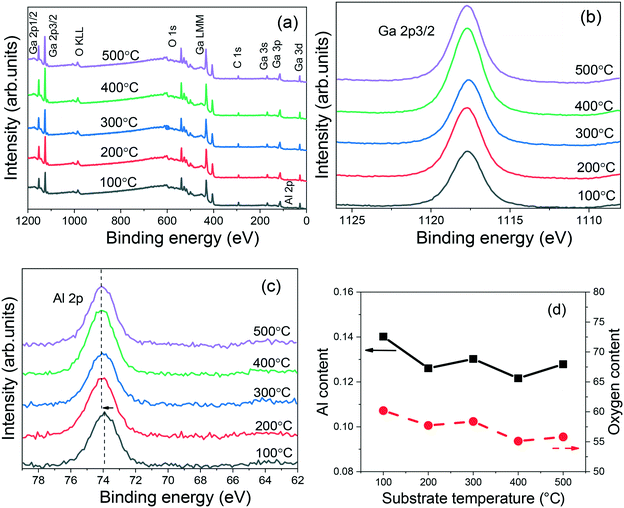 | ||
| Fig. 3 (a) XPS survey scans, (b) Ga 2p core level, (c) Al 2p core level and (d) Al and oxygen contents of (AlGa)2O3 films grown at different substrate temperatures by oxygen radical-assisted PLD. | ||
In order to investigate the crystal structure of the grown (AlGa)2O3 films, XRD patterns were recorded in the 2θ range of 10 to 70° as shown in Fig. 4. For the film grown at 100 °C, no obvious (AlGa)2O3 peak can be observed, indicating the amorphous nature of the film. For the film deposited at a substrate temperature of 200 °C, two obvious peaks located at 2θ values of about 38° and 59° have been observed, which has been ascribed to the (−402) and (−603) faces of monoclinic structured β-(AlGa)2O3. For the film deposited at a substrate temperature of 300 °C, one more peak located at 19° appears, which has been ascribed to the (−201) face of β-(AlGa)2O3. The existence of the sloping background that increases towards the small angles in the XRD patterns suggests that the films have amorphous components.27 Further increasing the substrate temperature to 400 and 500 °C caused the appearance of additional XRD peaks located at 31° and 45°, which can be ascribed to the (−401) and (−601) faces of the β-(AlGa)2O3 film. From the above results, it is obvious that (−201) oriented β-(AlGa)2O3 films have been obtained at substrate temperatures from 200 to 300 °C. The degradation of orientation for films grown at higher temperatures has been observed by several groups.28,29 Jones et al.28 reported the degradation of film orientation with substrate temperature. Increasing the substrate temperature will increase the surface mobility of the deposited atoms and tend to favor the development of an equilibrium structure.21,28,30 It is noticeable that the substrate temperature variation has caused simultaneous microstructure evolution and preferred orientation change as observed in Fig. 1 and 4. These variations are similar to radio frequency magnetron sputtered ZnO thin films reported by Lee et al.29 Films deposited at lower temperatures have a smooth structure with a high orientation, which is generally thought to be developed because of the low adatom mobility and a shadowing effect.31–33 It is clear that the adatoms already have suitable kinetic energy and mobility for growing highly oriented films at low substrate temperatures. Thus, the adatoms at high substrate temperatures may have sufficient adatom mobility to overcome the self-shadowing effect, resulting in the growth of large elongated grains with degraded orientation.29,34
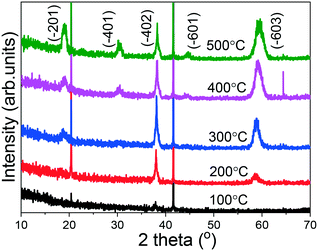 | ||
| Fig. 4 XRD patterns of (AlGa)2O3 films deposited at different substrate temperatures by oxygen radical-assisted PLD. | ||
The transmission spectra of the (AlGa)2O3 films deposited at different substrate temperatures are shown in Fig. 5(a). The presence of interference fringes indicates good surface homogeneity. All the films exhibit a high transmittance of over 90% in the ultraviolet and visible region. No multiple absorption edges, a typical characteristic of phase separation, can be observed for all the films. A sharp absorption edge near 230 nm, which is caused by the fundamental absorption of light for (AlGa)2O3 films, has been observed for all the films. The absorption edges of the (AlGa)2O3 films grown at temperatures from 200 °C to 500 °C are similar to each other while that of the film deposited at 100 °C has shifted toward a longer wavelength region. In order to determine the bandgap values of the (AlGa)2O3 films, the Tauc equation was applied: (αhν) = A(αhν − Eg)1/2, where Eg represents the bandgap energy, hν is the photon energy and α is the absorption coefficient. A linear character of (αhν) vs. hν has been observed in Fig. 5(b), confirming the direct transition type of the obtained (AlGa)2O3 films.25 By entrapping the linear part of the Tauc curves intercepting the energy axis (at αhν = 0), the bandgap values have been obtained. The obtained bandgap values of the (AlGa)2O3 films deposited at substrate temperatures from 200 to 500 °C are 5.34 eV while the bandgap value of the (AlGa)2O3 film deposited at 100 °C is 5.09 eV with uncertainties of 0.01 eV. We have investigated the evolution of the optical properties and band structures from amorphous to crystalline Ga2O3 films. We found an abrupt bandgap value variation when amorphous to crystalline transition took place.35 The bandgap variation of (AlGa)2O3 films in this work is similar to that of our previously reported Ga2O3 films; the bandgap value of the (AlGa)2O3 films changed abruptly when the substrate temperature was above 100 °C, at which amorphous to crystalline transition took place.
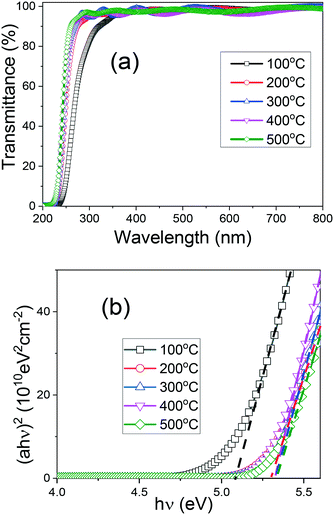 | ||
| Fig. 5 (a) Transmission spectra and (b) (αhν)2versus hν plots of (AlGa)2O3 films deposited at different substrate temperatures by oxygen radical-assisted PLD. | ||
From the above results, it is evidenced that the crystalline temperature for β-(AlGa)2O3 films grown by oxygen radical-assisted PLD can be as low as 200 °C. We have also deposited (AlGa)2O3 films by PLD without radical assistance at various substrate temperatures and found that the crystalline substrate temperature for the (AlGa)2O3 film is about 350 °C.23 Shao et al.15 found that the growth temperature of c-axis oriented ZnO films can be lowered to 100 °C using plasma-assisted pulsed laser deposition. Lim et al.36 found that the SiO2 films can be obtained at temperatures below 250 °C by plasma enhanced atomic layer deposition. The oxygen radical should benefit the low temperature growth of β-(AlGa)2O3 films by two aspects in this work: (1) providing reactive oxygen species for the formation of (AlGa)2O3 precursors through the reaction with the ablation species and (2) enhancing the mobility of the adatoms on the substrate surface, which could obtain enough energy to occupy energy-favored locations in the lattice for oriented crystallographic growth.15
Conclusions
(AlGa)2O3 films were grown on (0001) sapphire substrates by oxygen radical-assisted PLD. The prepared films show a good (−201) orientation perpendicular to the substrates even at a deposition temperature as low as 200 °C. All the films deposited from 100 to 500 °C show a high transmittance of over 90% in the UV and visible range. Abrupt bandgap value variation has been observed for films deposited at substrate temperatures higher than 100 °C, which agrees with the amorphous to crystalline transition temperature evidenced by XRD. The speed of the film thickness decrease with substrate temperature is much slower for (AlGa)2O3 films grown with oxygen radical assistance, indicating the suppression of the evaporation of volatile species with the help of oxygen radical species.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Japan Society for Promotion of Science (JSPS) for providing grants (KAKENHI Grant No. 19K04492 and. 18K04681) and the Partnership Project for Fundamental Technology Researches of the Ministry of Education, Culture, Sports, Science and Technology, Japan. One of the authors (Fabi Zhang) was sponsored by the Guangxi Scholarship Fund of Guangxi Education Department and the Guangxi Science and Technology Base and Talent Special Project (AD18281084).References
- B. W. Krueger, C. S. Dandeneau, E. M. Nelson, S. T. Dunham, F. S. Ohuchi and M. A. Olmstead, J. Am. Ceram. Soc., 2016, 99, 2467–2473 CrossRef CAS
.
- H. Okumura, Y. Kato, T. Oshima and T. Palacios, Jpn. J. Appl. Phys., 2019, 58, SBBD12 CrossRef CAS
.
- Q. Feng, X. Li, G. Han, L. Huang, F. Li, W. Tang, J. Zhang and Y. Hao, Opt. Mater. Express, 2017, 7, 1240–1248 CrossRef CAS
.
- S. Krishnamoorthy, Z. Xia, C. Joishi, Y. Zhang, J. McGlone, J. Johnson, M. Brenner, A. R. Arehart, J. Hwang, S. Lodha and S. Rajan, Appl. Phys. Lett., 2017, 111, 023502 CrossRef
.
- C.-C. Wang, S.-H. Yuan, S.-L. Ou, S.-Y. Huang, K.-Y. Lin, Y.-A. Chen, P.-W. Hsiao and D.-S. Wuu, J. Alloys Compd., 2018, 765, 894–900 CrossRef CAS
.
- T. Oshima, T. Okuno, N. Arai, Y. Kobayashi and S. Fujita, Jpn. J. Appl. Phys., 2009, 48, 070202 CrossRef
.
- E. Ahmadi, Y. Oshima, F. Wu and J. S. Speck, Semicond. Sci. Technol., 2017, 32, 035004 CrossRef
.
- K. Kaneko, K. Suzuki, Y. Ito and S. Fujita, J. Cryst. Growth, 2016, 436, 150–154 CrossRef CAS
.
- R. Miller, F. Alema and A. Osinsky, IEEE Trans. Semicond. Manuf., 2018, 31, 467–474 Search PubMed
.
- X. Wang, Z. Chen, F. Zhang, K. Saito, T. Tanaka, M. Nishio and Q. Guo, AIP Adv., 2016, 6, 015111 CrossRef
.
- R. Wakabayashi, T. Oshima, M. Hattori, K. Sasaki, T. Masui, A. Kuramata, S. Yamakoshi, K. Yoshimatsu and A. Ohtomo, J. Cryst. Growth, 2015, 424, 77–79 CrossRef CAS
.
- Q. Feng, Z. Feng, Z. Hu, X. Xing, G. Yan, J. Zhang, Y. Xu, X. Lian and Y. Hao, Appl. Phys. Lett., 2018, 112, 072103 CrossRef
.
- D. Huang, F. Liao, S. Molesa, D. Redinger and V. Subramanian, J. Electrochem. Soc., 2003, 150, G412–G417 CrossRef CAS
.
- V. Jousseaume, J. Cuzzocrea, N. Bernier and V. T. Renard, Appl. Phys. Lett., 2011, 98, 123103 CrossRef
.
- J. Shao, Y. Q. Shen, J. Sun, N. Xu, D. Yu, Y. F. Lu and J. D. Wu, J. Vac. Sci. Technol., B, 2008, 26, 214–218 CrossRef CAS
.
- K. Matsubara, P. Fons, K. Iwata, A. Yamada and S. Niki, Thin Solid Films, 2002, 422, 176–179 CrossRef CAS
.
- J. A. Thornton, J. Vac. Sci. Technol., A, 1975, 12, 830–835 CrossRef CAS
.
- V. Torrisi and F. Ruffino, Surf. Coat. Technol., 2017, 315, 123–129 CrossRef CAS
.
- S. Mukherjee and D. Gall, Thin Solid Films, 2013, 527, 158–163 CrossRef CAS
.
- Y. Y. Choi and D. J. Choi, CrystEngComm, 2013, 15, 6963–6970 RSC
.
- L. Yu, L. Zhang, H. Song, X. Jiang and Y. Lv, CrystEngComm, 2014, 16, 3331–3340 RSC
.
- F. B. Zhang, K. Saito, T. Tanaka, M. Nishio and Q. X. Guo, J. Cryst. Growth, 2014, 387, 96–100 CrossRef CAS
.
- X. Wang, Z. Chen, F. Zhang, K. Saito, T. Tanaka, M. Nishio and Q. Guo, Ceram. Int., 2016, 42, 12783–12788 CrossRef CAS
.
- T. Mathew, Y. Yamada, A. Ueda, H. Shioyama and T. Kobayashi, Appl. Catal., A, 2005, 286, 11–22 CrossRef CAS
.
- F. Zhang, K. Saito, T. Tanaka, M. Nishio, M. Arita and Q. Guo, Appl. Phys. Lett., 2014, 105, 162107 CrossRef
.
- Z. Hu, Q. Feng, J. Zhang, F. Li, X. Li, Z. Feng, C. Zhang and Y. Hao, Superlattices Microstruct., 2018, 114, 82–88 CrossRef CAS
.
- T. D. Shen, C. C. Koch, T. L. McCormick, R. J. Nemanich, J. Y. Huang and J. G. Huang, J. Mater. Res., 1995, 10, 139–148 CrossRef CAS
.
- M. I. Jones, I. R. McColl and D. M. Grant, Surf. Coat. Technol., 2000, 132, 143–151 CrossRef CAS
.
- Y. E. Lee, J. B. Lee, Y. J. Kim, H. K. Yang, J. C. Park and H. J. Kim, J. Vac. Sci. Technol., A, 1996, 14, 1943–1948 CrossRef CAS
.
- S. Maiti, S. Pal and K. K. Chattopadhyay, CrystEngComm, 2015, 17, 9264–9295 RSC
.
- F. Ruffino, I. Crupi, F. Simone and M. G. Grimaldi, Appl. Phys. Lett., 2011, 98, 023101 CrossRef
.
- J. A. Venables, G. D. T. Spiller and M. Hanbucken, Rep. Prog. Phys., 1984, 47, 399–459 CrossRef
.
- J. Carrey and J. L. Maurice, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245408 CrossRef
.
- P. Sundara Venkatesh and K. Jeganathan, CrystEngComm, 2014, 16, 7426–7433 RSC
.
- F. Zhang, H. Li, Y.-T. Cui, G.-L. Li and Q. Guo, AIP Adv., 2018, 8, 045112 CrossRef
.
- J. W. Lim, S. J. Yun and J. H. Lee, ETRI J., 2005, 27, 118–121 CrossRef
.
| This journal is © The Royal Society of Chemistry 2020 |
