Seeking a novel energetic co-crystal strategy through the interfacial self-assembly of CL-20 and HMX nanocrystals†
Menghua
Zhang
ab,
Yingxin
Tan
a,
Xu
Zhao
 b,
Jianhu
Zhang
b,
Shiliang
Huang
b,
Zhaohui
Zhai
c,
Yu
Liu
*b and
Zhijian
Yang
b,
Jianhu
Zhang
b,
Shiliang
Huang
b,
Zhaohui
Zhai
c,
Yu
Liu
*b and
Zhijian
Yang
 *b
*b
aSchool of Environment and Safety Engineering, North University of China, Taiyuan, 030051, China
bInstitute of Chemical Materials, China Academy of Engineering Physics, Mianyang, 621900, China. E-mail: liuyu307@caep.cn; zhijianyang@caep.cn
cInstitute of Fluid Physic and Microsystem & Terahertz Research center, CAEP, Mianyang, 621900, China
First published on 14th November 2019
Abstract
Energetic co-crystallization has been extensively explored as an effective strategy to balance the energy and sensitivity of energetic materials. In the present study, CL-20/HMX co-crystals with high purity, uniform morphology, well-proportioned size distribution, compact internal structure and reduced sensitivity were fabricated by a solvent-induced self-assembling approach using corresponding nanoparticles as the basic units. Such a CL-20/HMX co-crystal based on the self-assembly of a nano-explosive has been reported for the first time, providing a novel, high-yield, general and easily scaled-up method for the fabrication of energetic co-crystals. Notably, the formation processes of the as-synthesized co-crystals were determined as interfacial self-assembly at nanoparticle scale instead of the complete dissolution and nucleation process at the molecular level as observed with the traditional recrystallization technique. The completely formed CL-20/HMX co-crystal structure with few separate-assembled CL-20 or HMX crystals was charecterized by PXRD, TG-DSC, Raman, FT-IR, DG and SCXRD analyses. Additionally, the formation of intermolecular hydrogen bonds was confirmed by Terahertz (THz) analysis. The typical Avrami equation was constructed for modeling the kinetics of the assembly of CL-20/HMX co-crystals, and the self-assembly process can be generally summarized as the induction of nano-particles, oriented aggregation, surface integration and single co-crystal formation.
Introduction
As typical functional materials with high energy, energetic materials (EMs) have been widely applied in explosives, propellants, and pyrotechnics due to their outstanding combustion efficiency and high energy releasing rate.1,2 Nevertheless, potential applications of several nitroamine explosives, such as hexanitro-hexaazaiso-wurtzitane (CL-20), 1,3,5,7-tetranitro-1,3,5,7-tetrazacy-clooctane (HMX) and hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX),3 are still impeded by their high mechanical sensitivity. Taking CL-20 as an example, although it is known as the most typical high-energy density material (HEDM), with 14–20% higher energy than HMX,4 the application prospects are restricted due to its inherent high sensitivity. Therefore, various strategies have been adopted to improve the safety performance of EMs. First, the synthesis of new energetic crystal compounds, such as energetic salts, has recently been used as a direct approach to balance the sensitivity and detonation performance.5–7 However, the design and fabrication of high-performance compounds is usually time-consuming. In addition, improving the crystal quality by careful recrystallization is another method to reduce the sensitivity, attributing to the removed impurities and defects in crystals,3,8 but the desensitization effect is limited because of the unchanged components. Additionally, polymer-bonded explosives (PBX)9 have been extensively applied in the EM field to improve the performance of explosives. Unfortunately, due to the large quantity of inert polymers used, it will inevitably cause some energy loss.Nowadays, co-crystallization10,11 has become a novel technology, which depends on noncovalent interactions including hydrogen bonding,12 van der Waals forces and π–π stacking13–15 to efficiently combine independent molecule components; the obtained co-crystals have been successfully applied in pharmaceuticals16,17 and optics.18 Co-crystal technology for EMs has attracted great attention, and involves the combined rearrangement of different molecules to obtain a supramolecular structure, which significantly reduces mechanical sensitivity without scarifying a high amount of energy.19 During the past decade, CL-20,20,21 HMX,22,23 benzotrifuroxan (BTF),24,25 1,3,5-triamino-2,4,6-trinitrobenzene (TATB)26 and other EMs have been studied to gain co-crystals with high energy and reduced sensitivity. Matzger et al.27 first confirmed an energetic co-crystal composed of CL-20 and TNT with a molar ratio of 1:1, which maintains high energy and moderate sensitivity for its applications. Notably, the CL-20/HMX (ref. 28) co-crystal exhibits high detonation performance, which is close to that of CL-20, and similar sensitivity to HMX. Owing to the outstanding properties (detonation velocity, oxygen balance, thermal stability, mechanical sensitivity) of co-crystals, considerable efforts were devoted to exploring other routes to fabricate energetic co-crystals. The preparation of a co-crystal not only adopts crystallography methods, but also exploits engineering techniques, such as spray drying,29,30 spray flash evaporation (SFE)31 and rapid expansion of supercritical solution (RESS),32 which can be alternative ways to attain the desired performance. Unfortunately, the present fabrication of energetic co-crystals in high purity and high yield is difficult. Apart from this, the preparation process chiefly based on recrystallization also needs to be optimized and scaled-up. Therefore, a novel fabrication strategy for the energetic co-crystals needs to be developed to accelerate their development and application.
Recently, our group33 designed a solvent induced interfacial self-assembly strategy to obtain perfect energetic crystals in the particle scale, which was a bottom-up wet approach. The self-assembly behaviours and mechanism of a single explosive provide evident reference for the assembly of composites containing two energetic components. So far, there are few reports on the interfacial self-assembly of nano-sized energetic materials with multiple components,34 especially for the direct synthesis of energetic co-crystals. Furthermore, the interfacial self-assembly of nano-explosives at the nanoparticle scale, without complete dissolution and nucleation process,35 can experience oriented agglomeration that is the indispensable driving force in external stimulation and solvent induction.
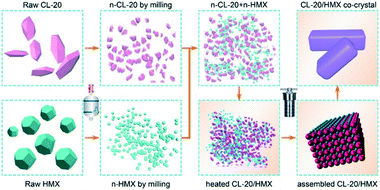
In this study, an interfacial self-assembly method has been adopted to fabricate a CL-20/HMX co-crystal of uniform size with regular morphology and few impurities. Apparently, the co-crystal was prepared at nanoparticle level rather than molecular level as compared with the traditional recrystallization method. Moreover, the method can be easily scaled up due to the minor influence of mass and heat transfer in the static assembly process. Systematic structural characterizations were performed to evaluate the morphology and physical properties of the as-prepared samples. The interfacial self-assembly method for explosives developed in this study could be generally extended in the development of other energy materials.
Experimental section
Chemicals and materials
CL-20 (ε-phase) was obtained by Liaoning Qing Yang Chemical Industry Co, Ltd., China. Fine CL-20 of about 500 nm diameter was obtained by mechanical grinding. HMX (β-phase) nanocrystals were furnished by the Nanjing University of Science and Technology, China. OP-10 Emulsifier (Nonylphenol polyethyleneoxy ether, 99%) was purchased from Macklin Co, Ltd., China. The other reagents were commercially obtained and used without further purification. The molecular structure of CL-20 and HMX is shown (Fig. S1) in the electronic ESI.†Preparation of CL-20/HMX co-crystal by self-assembly of nanocrystals
Raw ε-CL-20 (37.3 g) and β-HMX (12.6 g) nanocrystals with molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were produced by mechanical grinding in water to further decrease the particle size and obtain sufficient dispersion and blending of these two explosives, followed by centrifugation and freeze drying. Following this, the self-assembly of nano-sized CL-20 and HMX was conducted in a closed system, and experiment details are shown in the ESI.†
1 were produced by mechanical grinding in water to further decrease the particle size and obtain sufficient dispersion and blending of these two explosives, followed by centrifugation and freeze drying. Following this, the self-assembly of nano-sized CL-20 and HMX was conducted in a closed system, and experiment details are shown in the ESI.†
Characterizations
Scanning electron microscopy (SEM), optical polarizing microscope (OPM), power X-ray diffraction (PXRD), Fourier-transform infrared (FT-IR), Raman spectra, specific surface area analysis, thermogravimetry-differential scanning calorimetry (TG-DSC), density gradiometer (DG), and terahertz (THz) spectrum analysis were performed to study the structure and physicochemical properties of the samples. For the structure determination of the CL-20/HMX co-crystal, a single crystal prepared by the interfacial self-assembly strategy in pure H2O for 4 h was selected for structure determination by single-crystal X-ray diffraction (SCXRD). The quasi-static compression method33,36 was employed to evaluate the coherence strength of the as-prepared CL-20/HMX co-crystal. The impact sensitivity was determined and shown in the form of impact energy by a BAM method.33 Details of the characterization methods are shown in the ESI† document.Structure determination of co-crystal
A single crystal of CL-20/HMX co-crystals prepared by interfacial self-assembly in a pure water system was selected for structure determination by single-crystal X-ray diffraction (SCXRD). The crystal size is about 20 × 20 × 20 μm. The data were collected on a SuperNova diffractometer with CuKα (λ = 1.541783 Å) radiation at 293 K. Data reduction and empirical absorption correction were applied with CrysAlisPro. Due to the small crystal size, the quality of the diffraction data is not very high (Rint = 0.4098), but still good enough for solving the structure by SHELX.14. All non-hydrogen atoms can be directly located from the difference of Fourier map, which shows the same structure model as that in literature.28 However, the anisotropy thermal factors were not refined due to the limitation of the data quality. Crystallographic details of the structure refinement are given in Table 1. The atomic coordinates and equivalent isotropic displacement parameters can be found in the crystallographic information files (CCDC-1919565) viahttp://www.ccdc.cam.ac.uk/data__ request/cif.| Sample | CL-20/HMX co-crystal | Ref. 28 |
|---|---|---|
| Formula | C6H6N12O12·0.5(C4H8N8O8) | C6H6N12O12·0.5(C4H8N8O8) |
| Crystal system | Monoclinic | Monoclinic |
| a/Å | 16.537(4) | 16.3455(12) |
| b/Å | 10.001(4) | 9.9361(5) |
| c/Å | 12.295(3) | 12.1419(7) |
| α/° | 90° | 90° |
| β/° | 99.96(3)° | 99.233(7)° |
| γ/° | 90° | 90° |
| V/Å3 | 2002.6(11) | 1946.42(11) |
| T/K | 293(2) | 95 |
| Space group | P21/c | P21/c |
| Z | 4 | 4 |
| λ/Å | 1.54184 | 1.54187 |
| R int | 0.4098 | 0.0227 |
| R 1 | 0.1668 (I > σ(I)) 0.3876 (all data) | 0.0380 (I > σ(I)) |
| wR(F2) | 0.3910(I > 2σ(I)) 0.5023 (all data) | 0.1162(I > 2σ(I)) |
| Goodness of fit on F2 | 1.003 | 1.099 |
Results and discussion
Crystal morphology and structure of CL-20/HMX co-crystals
The CL-20/HMX co-crystal was successfully prepared by the solvent-induced interfacial self-assembly strategy using highly blended CL-20 and HMX nanocrystals as raw materials, and detailed synthesis procedures have been summarized in ESI.† Average grain size of raw CL-20, HMX nanocrystals and CL-20 + HMX (mechanical mixture in molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
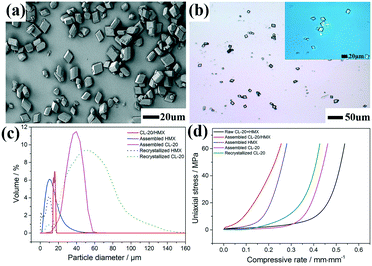
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) co-crystals can be observed to be 500 nm, 100 nm and 300 nm, respectively (SEM image, Fig. S2†). The morphology and structure information of the CL-20/HMX co-crystal after self-assembly in pure H2O for 4 h are displayed in Fig. 1. Clearly, the CL-20/HMX co-crystal (after hydrothermal treatment) exhibited a uniform polyhedral morphology of about 20 μm with a narrow particle size distribution in a reactor (Fig. 1a). Besides, the OPM images displayed highly transparent (top right inset in Fig. 1b) and compact polyhedral crystals with few defects, which might indicate a high quality of the CL-20/HMX co-crystals (Fig. 1b).
1) co-crystals can be observed to be 500 nm, 100 nm and 300 nm, respectively (SEM image, Fig. S2†). The morphology and structure information of the CL-20/HMX co-crystal after self-assembly in pure H2O for 4 h are displayed in Fig. 1. Clearly, the CL-20/HMX co-crystal (after hydrothermal treatment) exhibited a uniform polyhedral morphology of about 20 μm with a narrow particle size distribution in a reactor (Fig. 1a). Besides, the OPM images displayed highly transparent (top right inset in Fig. 1b) and compact polyhedral crystals with few defects, which might indicate a high quality of the CL-20/HMX co-crystals (Fig. 1b).
To further explore the CL-20/HMX co-crystal, the size distribution was determined and shown in Fig. 1c. Compared with the traditionally re-crystallized HMX and CL-20, the self-assembled CL-20 and HMX had an apparently narrow size-distribution with a grain size of 40.1 μm and 9.4 μm, respectively. Surprisingly, it was revealed that the CL-20/HMX co-crystals were characterized by a narrow size distribution (19.1 μm) than the individual components, which is consistent with the SEM results in Fig. 1a. A confined quasi-static compression method was adopted to gain the coherence strength and verify the internal structure of the CL-20/HMX co-crystal. Fig. 1d shows the curves of uniaxial stress versus compressive rate. In detail, the curves of the CL-20/HMX co-crystal and recrystallized CL-20 exhibited dissimilar compression behaviours, emphasizing that the relative crystal grain size was about 50 μm. The ISM (initial secant modulus) of the fine raw CL-20 + HMX mixture, assembled HMX and CL-20 was 70.6 MPa, 151.0 MPa and 78.6 MPa, respectively. Interestingly, the assembled CL-20/HMX co-crystal displayed an ISM value of 223.4 MPa, which was higher than that of recrystallized CL-20 (84.4 MPa) that was about 50 μm in diameter, implying a relatively compact internal structure for the assembled CL-20/HMX crystals. Moreover, the BET (Brunauer–Emmett–Teller) method was performed to evaluate the specific surface area of the as-assembled CL-20/HMX co-crystals (Table S1†). For raw CL-20 + HMX, CL-20 and HMX nano-crystals, the specific surface area reached 8.25 m2 g−1, 6.39 m2 g−1, and 8.52 m2 g−1, respectively, and decreased to 0.94 m2 g−1, 0.81 m2 g−1 and 0.91 m2 g−1, respectively, after the self-assembly, indicating the compact internal crystal structure of the as-prepared CL-20/HMX co-crystal, corresponding to the quasi-static compression result.
Additionally, the crystal density of the as-prepared CL-20/HMX co-crystal was further tested by a density gradiometer (DG), and the results are shown in Fig. S3 and Table S2.† As extended grinding time can result in a more uniform mixing and refining effect, CL-20 + HMX mixtures after grinding for 5 h and 10 h were prepared and selected as raw materials for comparison. Pipes 2 and 3 contain the same CL-20/HMX co-crystal samples from mechanical grinding for 10 h, which are different from the samples of pipe 6 by grinding for 5 h. Based on the same density gradient solution, the as-prepared CL-20/HMX co-crystals exhibit narrow density distribution. It is worth noting that since the size of the as-prepared sample was relatively small, crossing between pipes 2 and 3 occurred, but the co-crystals had a density of about 1.95 g cm−3 and displayed a narrow density distribution. In contrast, the co-crystals from pipe 6 had a wide density distribution with density ranging from 1.91 g cm−3 to 1.95 g cm−3 from top to bottom. In other words, the DG results indicated a uniform density of the as-prepared CL-20/HMX co-crystal.
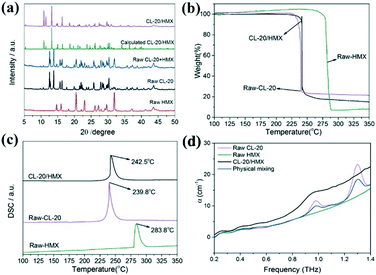
PXRD patterns of the CL-20/HMX co-crystal and raw materials are exhibited in Fig. 2a. The most intense peaks of raw CL-20 (12.6°, 13.8°, and 30.3°) and HMX (14.7°, 29.6°, and 31.9°) in these patterns can be indexed to the typical and stable ε-CL-20 and β-HMX phase in ambient enviorment.37,38 The diffraction peaks of raw CL-20 + HMX (physical mixture, slightly ground samples) were the superposition of those of ε-CL-20 and β-HMX, and no new peaks appeared, confirming that the raw materials could not appear in the co-crystal before assembly.39 Additionally, the typical diffraction peaks of the CL-20/HMX co-crystal at 10.9°, 11.5° and 13.2° are visible after the 2 h hydrothermal reaction for, consistent with the CL-20/HMX co-crystal phase as previously reported.28 Furthermore, the characteristic peaks of both raw materials CL-20 and HMX disappear in the pattern of the co-crystal product, indicating that the formation of the CL-20/HMX co-crystal with high chemical purity could be achieved by such an interfacial self-assembly approach.
Raman spectroscopy tests were performed for the raw materials and as-prepared samples to investigate the formation mechanism of the CL-20/HMX co-crystal.28 As shown in Fig. S4,† the peaks in the Raman spectra of the CL-20/HMX co-crystal underwent a red or blue shift. The symmetric stretching vibration of the –NO2 group and asymmetric stretching vibration of the NO2 group in CL-20 and the telescopic vibration of the C–H bond in HMX can be indexed at 1381.7–1247.9 cm−1, 1596.0–1578.1 cm−1 and 2993.2 cm−1, respectively. The asymmetric stretching vibration of the NO2 group and telescopic vibration of the C-H bond of the CL-20/HMX co-crystal appeared at 1594.4–1557.2 cm−1 and 3006.6 cm−1, respectively. The Raman shift results of this co-crystal may be attributed to the hydrogen bond interactions between –NO2 in CL-20 and C–H bond in HMX.14 Similarly, FT-IR spectra (Fig. S5†) further confirm the structure of the raw materials and CL-20/HMX co-crystal.38 Clearly, the symmetric stretching vibration of the NO2 group and asymmetric stretching vibration of the NO2 group of CL-20 and the C–H stretching vibration of HMX have shifted from 1382.7–1284.4 cm−1, 1608.3–1565.9 cm−1 and 3037.4–3025.8 cm−1 to 1396.2–1274.7 cm−1, 1602.6–1525.4 cm−1 and 3031.6–3016.1 cm−1, respectively, corresponding to the XRD results in Fig. 2a. A large number of hydrogen bonds between CL-20 and HMX lead to the formation of a stable CL-20/HMX co-crystal structure.40 These hydrogen bonds alternately connect CL-20 and HMX molecules to form the chains and propagate through intermolecular interactions to form a co-crystal structure in the unit cell.30 Moreover, the single crystal X-ray diffraction (SCXRD) data (Table 1) further confirm the hydrogen bonding between the as-synthesized CL-20/HMX cocrystals.
Additionally, intermolecular interactions can alter the physicochemical properties of crystals. To probe the thermal properties of the CL-20/HMX co-crystal, the TG-DSC results are shown in Fig. 2b and c. Typically, the onset temperature and peak temperature of the as-prepared CL-20/HMX co-crystal can be observed at 225.6 °C and 242.5 °C, respectively. In addition, only a one-step decomposition with the steep slope up to the peak temperature has been achieved. The similar decomposition behavior of the co-crystal with CL-20 might be attributed to the predominant content of CL-20 in the CL-20/HMX co-crystal.40 Moreover, the thermal decomposition result of physical mixing (molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is shown in Fig. S6;† two decomposition temperatures are observed at 236.6 °C and 281.6 °C, corresponding to the decomposition CL-20 and HMX. It is clear that the DSC curve of the as-prepared CL-20/HMX co-crystal exhibits one distinct exothermic peak, which is different from the raw materials and physical mixing samples. This result further verified that the CL-20/HMX co-crystal achieved by such an interfacial self-assembly method was in the pure phase, which is consistent with XRD patterns in Fig. 2a. The THz spectrum has been proved as an effective characterization technique to verify the intermolecular interactions in the as-prepared CL-20/HMX, CL-20 and HMX; the corresponding results are shown in Fig. 2d. Compared with CL-20 and HMX, the peaks at 1.29 THz for CL-20/HMX co-crystal disappear. Apparently, CL-20/HMX exhibits absorption peaks that are different from those for the physical mixture at 0.92 THz, implying that hydrogen bonds14 were generated between CL-20 and HMX. Furthermore, the SCXRD result showed a solid evidence of the formation of the CL-20/HMX co-crystal after the interfacial self-assembly of the CL-20/HMX nanocrystal, and the detailed results are displayed in Table 1. Clearly, it is praiseworthy that the strategy of interfacial self-assembly is a novel, facile and general method for the fabrication the CL-20/HMX co-crystal. The as-prepared CL-20/HMX co-crystals exhibit well-defined size, high density, few impurities and excellent mechanical properties. In addition, this strategy might be favourable to improve safety performance and further enrich the crystallographic route.
1) is shown in Fig. S6;† two decomposition temperatures are observed at 236.6 °C and 281.6 °C, corresponding to the decomposition CL-20 and HMX. It is clear that the DSC curve of the as-prepared CL-20/HMX co-crystal exhibits one distinct exothermic peak, which is different from the raw materials and physical mixing samples. This result further verified that the CL-20/HMX co-crystal achieved by such an interfacial self-assembly method was in the pure phase, which is consistent with XRD patterns in Fig. 2a. The THz spectrum has been proved as an effective characterization technique to verify the intermolecular interactions in the as-prepared CL-20/HMX, CL-20 and HMX; the corresponding results are shown in Fig. 2d. Compared with CL-20 and HMX, the peaks at 1.29 THz for CL-20/HMX co-crystal disappear. Apparently, CL-20/HMX exhibits absorption peaks that are different from those for the physical mixture at 0.92 THz, implying that hydrogen bonds14 were generated between CL-20 and HMX. Furthermore, the SCXRD result showed a solid evidence of the formation of the CL-20/HMX co-crystal after the interfacial self-assembly of the CL-20/HMX nanocrystal, and the detailed results are displayed in Table 1. Clearly, it is praiseworthy that the strategy of interfacial self-assembly is a novel, facile and general method for the fabrication the CL-20/HMX co-crystal. The as-prepared CL-20/HMX co-crystals exhibit well-defined size, high density, few impurities and excellent mechanical properties. In addition, this strategy might be favourable to improve safety performance and further enrich the crystallographic route.
As the production capacity is very important, especially for the batch reactions as compared to the continuous reactions, the scaled-up preparation of the CL-20/HMX co-crystal was also performed, as shown in Fig. S7.† By using several stainless-steel reactors in the same time, 226.8 g of CL-20/HMX co-crystals was successfully obtained in three days, and this amount can be easily doubled if two ovens are adopted. Therefore, the batch technique used in this study is more efficient in production capacity than traditional batch reactions, attributing to the static assembly process without any agitation or other experimental actions.
Proposed mechanism for the assembly of CL-20/HMX co-crystal
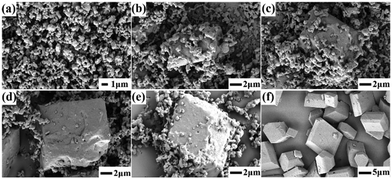 | ||
| Fig. 3 SEM images of CL-20/HMX at various assembly processes at 90 °C: (a) 5 min, (b) 20 min, (c) 40 min, (d) 1 h, (e) 2 h, and (f) 4 h. | ||
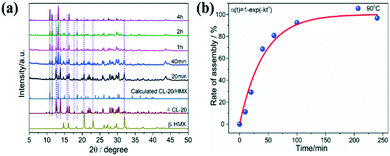 | ||
| Fig. 5 (a) XRD patterns and (b) kinetics curve of CL-20/HMX co-crystal at different reaction time in pure H2O. | ||
It should be noted that higher temperature could enhance the assembly rate and hence, it is difficult to grasp the intermediate products by real-time sampling and gain the assembly kinetics at relatively high temperature. Fortunately, we were successful in capturing the intermediates at 90 °C, which was a typical point of the assembled particles that can be detected distinctly. We not only obtained a complete assembly process, but also had enough time to study the intermediate crystal state to explore the process of assembly kinetics. Different from single components, the formation of the CL-20/HMX co-crystal involves a two-step evolution (rapid growth and interfacial periods of integration) in the kinetic curve (Fig. 5b). The kinetic data can be fitted using the Avrami (Johnson–Mehl) model:44
| α(t) = a × (1 − e−ktn) | (1) |
Nanoparticle self-assembly is a novel strategy to obtain CL-20/HMX co-crystals, indicating that it is a promising technique to resolve the contradictions in energy and sensitivity nature of the materials. The results of the impact energy are listed in Table S3.† CL-20/HMX co-crystals after assembly exhibit a desensitization effect. For HMX obtained by recrystallization, the impact energy was 5.5 J, and the sensitivity value for recrystallized CL-20 with size of about 50 μm was 3 J. In contrast, the impact energy of the CL-20/HMX co-crystal corresponding to 4.5 J was measured.45,46 Thus, the impact energy of the CL-20/HMX co-crystal is significantly higher than that of raw CL-20, indicating that energy close to that of HMX was gained, while the sensitivity was reduced by the self-assembly method. Moreover, uniform morphology and few defects may enhance the impact energy of the CL-20/HMX co-crystal. Therefore, the impact energy of the CL-20/HMX co-crystals increases due to interfacial self-assembly strategy, providing a facile and appreciable advantage in the field of EMs.
Conclusions
In summary, an interfacial self-assembly strategy was reported for the first time as a novel route to fabricate CL-20/HMX energetic co-crystals. The formation processes were investigated at a particle level, which are significantly different from the traditional solvent/non-solvent crystallization occurring at a molecular level. There was no dissolution or complete nucleation process at the nano-scale. The as-prepared CL-20/HMX co-crystals possessed uniform morphology, well-proportioned size distribution, high density, few impurities or defects, characteristic thermal performance and improved safety performance. The as-proposed method in this study was facile, general and environmentally friendly, and could be extended to the preparation of other energetic co-crystals. More importantly, it is a static assembly reaction without stirring. Therefore, this method can be influenced very slightly by the mass and heat transfer, facilitating its scale-up.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC 11502243, 21875232, 21875229).Notes and references
- S. B. Kim, K. J. Kim, M. H. Cho, J. H. Kim, K. T. Kim and S. H. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 9405–9412 CrossRef CAS.
- T. W. Myers, J. A. Bjorgaard, K. E. Brown, D. E. Chavez, S. K. Hanson, R. J. Scharff, S. Tretiak and J. M. Veauthier, J. Am. Chem. Soc., 2016, 138, 4685–4692 CrossRef CAS.
- A. E. D. M. Van Der Heijden and R. H. B. Bouma, Cryst. Growth Des., 2004, 4, 999–1007 CrossRef CAS.
- Y. Bayat and V. Zeynali, J. Energ. Mater., 2011, 29, 281–291 CrossRef CAS.
- Q. Ma, S. Huang, H. Lu, F. Nie, L. Liao, G. Fan and J. Huang, Cryst. Growth Des., 2019, 19, 714–723 CrossRef CAS.
- C. Zhang, C. Sun, B. Hu, C. Yu and M. Lu, Science, 2017, 355, 374–376 CrossRef CAS PubMed.
- J. Zhang, Q. Zhang, T. Vo, D. Parrish and J. Shreeve, J. Am. Chem. Soc., 2015, 137, 1697–1704 CrossRef CAS.
- A. R. Klapwijk, E. Simone, Z. K. Nagy and C. C. Wilson, Cryst. Growth Des., 2016, 16, 4349–4359 CrossRef CAS.
- Q. L. Yan, M. Gozin, F. Q. Zhao, A. Cohen and S. P. Pang, Nanoscale, 2016, 8, 4799–4851 RSC.
- D. I. A. Millar, H. E. Maynard-Casely, D. R. Allan, A. R. Lennie, A. J. Mackay, I. D. H. Oswald, C. C. Tang and C. R. Pulham, CrystEngComm, 2012, 14, 3742–3749 RSC.
- K. B. Landenberger and A. J. Matzger, Cryst. Growth Des., 2010, 10, 5341–5347 CrossRef CAS.
- (a) B. Duan, Y. Shu, N. Liu, B. Wang, X. Lu and Y. Lu, CrystEngComm, 2018, 20, 5790–5800 RSC; (b) J. Zhang and J.-M. Shreeve, CrystEngComm, 2016, 18, 6124–6133 RSC.
- Y. Liu, S. Li, J. Xu, H. Zhang, Y. Guan, H. Jiang, S. Huang, H. Huang and Z. Wang, Cryst. Growth Des., 2018, 18, 1940–1943 CrossRef CAS.
- C. Zhang, Z. Yang, X. Zhou, C. Zhang, Y. Ma, J. Xu, Q. Zhang, F. Nie and H. Li, Cryst. Growth Des., 2014, 14, 3923–3928 CrossRef CAS.
- R. Thakuria, N. K. Nath and B. K. Saha, Cryst. Growth Des., 2019, 19, 523–528 CrossRef CAS.
- S. G. Fleischman, S. S. Kuduva, J. A. McMahon, B. Moulton, R. D. B. Walsh, N. Rodríguez-Hornedo and M. J. Zaworotko, Cryst. Growth Des., 2003, 3, 909–919 CrossRef CAS.
- M. Gryl, T. Seidler, K. Stadnicka, I. Matulková, I. Nĕmec, N. Tesařová and P. Nĕmec, CrystEngComm, 2014, 16, 5765–5768 RSC.
- F. Pan, M. S. Wong, V. Gramlich, C. Bosshard and P. Günter, J. Am. Chem. Soc., 1996, 118, 6315–6316 CrossRef CAS.
- G. Liu, H. Li, R. Gou and C. Zhang, Cryst. Growth Des., 2018, 18, 7065–7078 CrossRef CAS.
- M. Ghosh, A. K. Sikder, S. Banerjee and R. G. Gonnade, Cryst. Growth Des., 2018, 18, 3781–3793 CrossRef CAS.
- N. Liu, B. Duan, X. Lu, H. Mo, Q. Zhang and B. Wang, CrystEngComm, 2018, 20, 2060–2067 RSC.
- K. B. Landenberger and A. J. Matzger, Cryst. Growth Des., 2012, 12, 3603–3609 CrossRef CAS.
- C. Hou, Y. Zhang, Y. Chen, X. Jia, S. Zhang and Y. Tan, Propellants, Explos., Pyrotech., 2018, 43, 916–922 CrossRef CAS.
- Z. Yang, H. Li, X. Zhou, C. Zhang, H. Huang, J. Li and F. Nie, Cryst. Growth Des., 2012, 12, 5155–5158 CrossRef CAS.
- H. Zhang, C. Guo, X. Wang, J. Xu, X. He, Y. Liu, X. Liu, H. Huang and J. Sun, Cryst. Growth Des., 2013, 13, 679–687 CrossRef CAS.
- H. Xu, X. Duan, H. Li and C. Pei, RSC Adv., 2015, 5, 95764–95770 RSC.
- O. Bolton and A. Matzger, Angew. Chem., Int. Ed., 2011, 50, 8960–8963 CrossRef CAS.
- O. Bolton, L. R. Simke, P. F. Pagoria and A. J. Matzger, Cryst. Growth Des., 2012, 12, 4311–4314 CrossRef CAS.
- H. Li, C. An, W. Guo, X. Geng, J. Wang and W. Xu, Propellants, Explos., Pyrotech., 2015, 40, 652–658 CrossRef CAS.
- B. Gao, D. Wang, J. Zhang, Y. Hu, J. Shen, J. Wang, B. Huang, Z. Qiao, H. Huang, F. Nie and G. Yang, J. Mater. Chem. A, 2014, 2, 19969–19974 RSC.
- D. Spitzer, B. Risse, F. Schnell, V. Pichot, M. Klaumünzer and M. R. Schaefer, Sci. Rep., 2014, 4, 4–9 Search PubMed.
- B. Siegert, M. Comet and D. Spitzer, Nanoscale, 2011, 3, 3534–3544 RSC.
- Z. Yang, F. Gong, G. He, Y. Li, L. Ding, F. Nie and F. Huang, Cryst. Growth Des., 2018, 18, 1657–1665 CrossRef CAS.
- S. Wintzheimer, T. Granath, M. Oppmann, T. Kister, T. Thai, T. Kraus, N. Vogel and K. Mandel, ACS Nano, 2018, 12, 5093–5120 CrossRef CAS PubMed.
- (a) S. R. Challa, A. T. Delariva, T. W. Hansen, S. Helveg, J. Sehested, P. L. Hansen, F. Garzon and A. K. Datye, J. Am. Chem. Soc., 2011, 133, 20672–20675 CrossRef CAS; (b) X. Zhao, W. Cai, Y. Yang, X. Song, Z. Neale, H.-E. Wang, J. Sui and G. Cao, Nano Energy, 2018, 47, 224–234 CrossRef CAS.
- L. Ming, H. Ming, K. Bin, W. Maoping, L. Hongzhen and X. Rong, Propellants, Explos., Pyrotech., 2007, 32, 401–405 CrossRef CAS.
- L. Pu, J. J. Xu, X. F. Liu and J. Sun, J. Energ. Mater., 2016, 34, 205–215 CrossRef CAS.
- C. An, H. Li, B. Ye and J. Wang, J. Nanomater., 2017, 8, 1–7 CrossRef.
- H. Qiu, R. B. Patel, R. S. Damavarapu and V. Stepanov, CrystEngComm, 2015, 17, 4080–4083 RSC.
- C. Zhang, X. Xue, Y. Cao, J. Zhou, A. Zhang, H. Li, Y. Zhou, R. Xu and T. Gao, CrystEngComm, 2014, 16, 5905–5916 RSC.
- A. J. Bourque and G. C. Rutledge, Macromolecules, 2016, 49, 3956–3964 CrossRef CAS.
- M. Iggland and M. Mazzotti, Cryst. Growth Des., 2012, 12, 1489–1500 CrossRef CAS.
- C. Huaxiong, C. Shusen, L. Lijie and J. Shaohua, Propellants, Explos., Pyrotech., 2008, 33, 467–471 CrossRef.
- R. Wang, S. Zou, B. Jiang, B. Fan, M. Hou, B. Zuo, X. Wang, J. Xu and Z. Fan, Cryst. Growth Des., 2017, 11, 5908–5917 CrossRef.
- C. Zhang, X. Xue, Y. Cao, J. Zhou, A. Zhang, H. Li, Y. Zhou, R. Xu and T. Gao, CrystEngComm, 2013, 15, 4003–4014 RSC.
- Y. Liu, S. Niu, W. Lai, T. Yu, Y. Ma, H. Gao, F. Zhao and Z. Ge, CrystEngComm, 2019, 21, 4910–4917 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 1919565. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ce01447k |
| This journal is © The Royal Society of Chemistry 2020 |