Open Access Article
Open Access ArticleFacile gas-phase hydrodeoxygenation of 2,5-dimethylfuran over bifunctional metal-acid catalyst Pt–Cs2.5H0.5PW12O40†
Hanan
Althikrallah
 ab,
Elena F.
Kozhevnikova
a and
Ivan V.
Kozhevnikov
ab,
Elena F.
Kozhevnikova
a and
Ivan V.
Kozhevnikov
 *a
*a
aDepartment of Chemistry, University of Liverpool, Liverpool L69 7ZD, UK. E-mail: kozhev@liverpool.ac.uk
bDepartment of Chemistry, King Faisal University, College of Science, P.O. Box 400, Al-Ahsa 31982, Saudi Arabia. E-mail: halhekrallh@kfu.edu.sa
First published on 24th November 2020
Abstract
2,5-Dimethylfuran is deoxygenated to n-hexane with 100% yield on a bifunctional Pt/C–Cs2.5H0.5PW12O40 catalyst under very mild conditions (90 °C, 1 bar H2) in a one-step gas-phase process. A proposed mechanism includes a sequence of hydrogenolysis, hydrogenation and dehydration steps occurring on Pt and proton sites of the bifunctional catalyst.
A biomass-derived furanic compound is a low-cost renewable feedstock, which can be converted to green fuels and a wide range of value-added chemicals by catalytic hydroconversion.1–9 Hydrodeoxygenation (HDO) is an effective strategy to produce fuels from renewable feedstock. Much current research is focussed on HDO of furanic compounds using heterogeneous catalysis4,5,7,9 and references therein. Complete oxygen removal from furanic compounds over noble metals to produce hydrocarbons requires harsh conditions (200–400 °C, 7–20 MPa H2 pressure).4,5,7 Bifunctional metal-acid catalysis has been found to be more efficient than monofunctional metal catalysis for the HDO of organic oxygenates such as ketones, alcohols, phenols, ethers and esters.10–14 Previously, this group has reported highly efficient bifunctional catalysts comprising platinum on acidic supports such as zeolite HZSM-510 and Keggin-type heteropoly acids11,12 for HDO of a wide range of aliphatic and aromatic ketones in the gas phase under mild conditions.10–12 This approach may be applicable to the HDO of furanic compounds. In hydroconversion of furanic compounds, Pt is selective to ring-opened products such as ketones and alcohols, whereas Pd, Rh and Ru have high selectivity to ring-saturated tetrahydrofuran derivatives.2,6,8 This is the result of a different bonding of furanic compounds to these metals.2,3
Here, the HDO of 2,5-dimethylfuran (DMF), chosen as a model for biomass-derived furanic compounds, is investigated in the presence bifunctional metal-acid catalysts comprising carbon-supported Pt, Pd, Ru and Rh together with acidic Cs salt of Keggin-type tungstophosphoric heteropoly acid Cs2.5H0.5PW12O40 (CsPW). Attention is focussed on the Pt–CsPW catalyst because, among these metals, Pt is the most active one for furan ring opening.2,8 CsPW is well documented as a solid Brønsted acid catalyst.15,16 It has important advantages over the parent heteropoly acid H3PW12O40 (HPW) in terms of much larger surface area, higher thermal stability (ca. 500 °C decomposition temperature) and high tolerance to water, with proton sites almost as strong as those in HPW.15,16 Previous H2-TPR, XRD and FTIR studies have shown that CsPW in Pt/CsPW and Pd/CsPW catalysts is resistant toward reduction by H2 below 600 °C, and the Keggin structure of CsPW is retained in CsPW-supported Pt and Pd catalysts after H2 treatment at 400 °C.17 Therefore, these catalysts should be stable under the rather mild reaction conditions applied in this work. The HDO reaction is studied both in gas and liquid phases, with the main emphasis on the gas-phase process. The gas-phase HDO is expected to be more efficient and environmentally benign compared to the liquid-phase process due to the absence of solvent, continuous rather than batch operation and easy product separation. It is demonstrated that the gas-phase HDO of DMF over Pt–CsPW is indeed a facile reaction, yielding 100% n-hexane under very mild conditions.
Table 1 shows representative results on the gas-phase HDO of DMF over bifunctional metal–CsPW catalysts (1% metal loading per total catalyst weight) at 70–100 °C and 1 bar H2 pressure. CsPW alone was not active under such conditions (entry 1). Physical mixtures of all carbon-supported metals with CsPW exhibited high catalytic activity, giving >99% DMF conversion. However, only Pt/C + CsPW gave ∼100% selectivity to n-hexane at full DMF conversion (entry 3 and 4) showing stable product selectivity for more than 5 h time on stream (TOS) (Fig. 1). Without CsPW present, Pt/C + SiO2 at the same Pt loading also gave >99% DMF conversion but yielded 2-hexanol as the main product (74% selectivity, entry 5) rather than hexane. Hence, for the efficient HDO, the presence of both Pt and proton sites in the catalyst is essential. In contrast to Pt, other noble metals (Pd, Rh and Ru) that are inactive in opening furan rings exhibited poor activity in the HDO reaction under the conditions applied (entries 10–19). These metals exhibited high selectivity towards ring hydrogenation to form 2,5-dimethyltetrahydrofuran (DMTHF) (a mixture of cis and trans isomers, cis/trans = 6–10), with or without the presence of CsPW (cf. Pd/C + SiO2, entry 13). Therefore, the ability of Pt to break the furan ring is the key to the deoxygenation of DMF. The Pt–CsPW catalyst operated under very mild conditions (70–100 °C, 1 bar H2) giving n-hexane in 100% yield. Importantly, no n-hexane isomerisation was observed under such conditions, thus allowing complete transformation of DMF to alkane without carbon backbone alteration. The isomerisation of n-hexane on Pt–CsPW has been reported to proceed at higher temperatures, >150 °C.18 These results show that the bifunctional catalyst Pt–CsPW is much more efficient than the monofunctional Pt catalysts reported previously.4,5,7
| Entry | Catalyst | Temperature (°C) | Conversionb (%) | Product selectivityb (% mol) | ||||
|---|---|---|---|---|---|---|---|---|
| Hexane | DMTHF | 2-Hexanone | 2-Hexanol | Other | ||||
a 1.6 kPa DMF, 20 ml min−1 H2 flow rate, 4 h TOS, 0.20 g catalyst.
b Average conversion and product selectivity over 4 h TOS.
c 9.6% Pt/C + CsPW (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 w/w, 20 mg Pt/C, 1% Pt loading per total catalyst weight).
d 9.6% Pt/C + SiO2 (1 9 w/w, 20 mg Pt/C, 1% Pt loading per total catalyst weight).
d 9.6% Pt/C + SiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 w/w, 20 mg Pt/C, 1% Pt loading).
e Catalyst prepared by impregnating H2PtCl6 from aqueous solution.
f Catalyst prepared by impregnating Pt(acac)2 from benzene solution.
g 7.8% Pd/C + CsPW (1 9 w/w, 20 mg Pt/C, 1% Pt loading).
e Catalyst prepared by impregnating H2PtCl6 from aqueous solution.
f Catalyst prepared by impregnating Pt(acac)2 from benzene solution.
g 7.8% Pd/C + CsPW (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w, 25 mg Pd/C, 1% Pd loading).
h 7.8% Pd/C + SiO2 (1 7 w/w, 25 mg Pd/C, 1% Pd loading).
h 7.8% Pd/C + SiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w, 25 mg Pd/C, 1% Pd loading).
i 3.0% Ru/C + CsPW (1 7 w/w, 25 mg Pd/C, 1% Pd loading).
i 3.0% Ru/C + CsPW (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w, 67 mg Ru/C, 1% Ru loading).
j 4.0% Rh/C + CsPW (1 2 w/w, 67 mg Ru/C, 1% Ru loading).
j 4.0% Rh/C + CsPW (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w, 50 mg Rh/C, 1% Rh loading). 3 w/w, 50 mg Rh/C, 1% Rh loading).
|
||||||||
| 1 | CsPW | 70 | 0 | |||||
| 2 | Pt/C + CsPWc | 70 | >99 | 68.1 | 13.0 | 0.1 | 16.4 | 2.3 |
| 3 | Pt/C + CsPWc | 90 | >99 | 99.6 | 0.3 | 0.0 | 0.1 | 0.0 |
| 4 | Pt/C + CsPWc | 100 | >99 | 99.9 | 0.1 | 0.0 | 0.0 | 0.0 |
| 5 | Pt/C + SiO2d | 90 | >99 | 8.8 | 10.7 | 2.1 | 74.2 | 4.2 |
| 6 | 1% Pt/CsPW-we | 70 | >99 | 61.2 | 30.7 | 0.3 | 6.5 | 1.1 |
| 7 | 1% Pt/CsPW-we | 90 | >99 | 98.4 | 1.2 | 0.0 | 0.1 | 0.3 |
| 8 | 1% Pt/CsPW-bf | 70 | >99 | 59.1 | 37.2 | 0.0 | 3.1 | 0.5 |
| 9 | 1% Pt/CsPW-bf | 90 | >99 | 99.6 | 0.1 | 0.0 | 0.0 | 0.3 |
| 10 | Pd/C + CsPWg | 70 | >99 | 0.2 | 98.8 | 0.1 | 0.0 | 0.9 |
| 11 | Pd/C + CsPWg | 90 | >99 | 1.5 | 97.4 | 0.3 | 0.0 | 0.8 |
| 12 | Pd/C + CsPWg | 100 | >99 | 2.8 | 93.5 | 0.9 | 0.1 | 2.7 |
| 13 | Pd/C + SiO2h | 70 | >99 | 0.0 | 99.8 | 0.0 | 0.0 | 0.2 |
| 14 | Ru/C + CsPWi | 70 | 98 | 7.2 | 89.8 | 0.4 | 2.1 | 0.6 |
| 15 | Ru/C + CsPWi | 90 | >99 | 13.9 | 84.7 | 0.1 | 0.3 | 0.6 |
| 16 | Ru/C + CsPWi | 100 | 98 | 16.1 | 78.7 | 0.8 | 0.6 | 3.8 |
| 17 | Rh/C + CsPWj | 70 | >99 | 1.4 | 97.5 | 0.9 | 0.1 | 0.2 |
| 18 | Rh/C + CsPWj | 90 | >99 | 8.7 | 91.1 | 0.0 | 0.1 | 0.1 |
| 19 | Rh/C + CsPWj | 100 | >99 | 17.4 | 82.3 | 0.03 | 0.02 | 0.2 |
 | ||
Fig. 1 Time course for HDO of DMF: 0.20 g catalyst 9.6% Pt/C + CsPW (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 w/w, 20 mg Pt/C, 1% Pt loading), 70 °C, 1.6 kPa DMF, 20 ml min−1 H2 flow rate. 9 w/w, 20 mg Pt/C, 1% Pt loading), 70 °C, 1.6 kPa DMF, 20 ml min−1 H2 flow rate. | ||
Reaction kinetics was examined at 80 °C (see the ESI,† for details). The HDO reaction was found to be zero-order in DMF at 1.6–6.8 kPa DMF partial pressure (Fig. S1, ESI†). This indicates that catalyst active sites were saturated with DMF. The reaction was first-order in the Pt/C catalyst (Fig. S2, ESI†). The same reaction orders have been reported for the gas- and liquid-phase hydroconversion of DMF in the presence of monofunctional catalyst Pt/C.2,8 The activation energy was estimated to be E = 65 kJ mol−1 in the temperature range of 70–90 °C, which is also close to the value 59 kJ mol−1 for the gas-phase reaction in the presence of Pt/C.8 The high E value and zero reaction order in DMF indicate no diffusion limitations in the HDO reaction over Pt–CsPW under the conditions studied.
Supported bifunctional catalysts Pt/CsPW-w and Pt/CsPW-b prepared by impregnating CsPW with H2PtCl6 from water and with Pt(acac)2 from benzene, respectively, showed practically the same performance in DMF deoxygenation as the Pt/C + CsPW physical mixture (Table 1, entries 6–9). This indicates that the reaction is not limited by migration of reaction intermediates between metal and acid sites in bifunctional catalysts since Pt and H+ active sites in the mixed Pt/C + CsPW catalysts are much more distant from each other than in the supported Pt/CsPW catalysts (Weisz intimacy criterion19).
Table 2 shows the effect of the Pt support (carbon, SiO2 and γ-Al2O3) on the activity of Pt–CsPW catalysts in HDO of DMF at 70 °C under kinetic control (DMF conversion <100%). From these results, the total turnover frequencies (TOF) per Pt site were calculated using the number of surface Pt sites in the catalysts from Pt dispersion (Table S1, ESI†). The turnover activity of the Pt sites decreases in the order of supports: C (9.3) > SiO2 (2.3) > γ-Al2O3 (0.42), where in round brackets are the TOF values (s−1) at 70 °C.
| Catalystb | Conversion (%) | TOF (s−1) | Product selectivity (% mol) | ||||
|---|---|---|---|---|---|---|---|
| Hexane | DMTHF | 2-Hexanone | 2-Hexanol | Other | |||
| a Pt/support (2.0 mg) + CsPW (0.19 g), 70 °C, 1.6 kPa DMF partial pressure, 40 ml min−1 H2 flow rate, 1 h TOS. b In round brackets the number of surface Pt sites in catalysts (μmol) calculated from Pt dispersion (Table S1). | |||||||
| 9.6% Pt/C + CsPW (0.038) | 79.4 | 9.3 | 46.5 | 16.0 | 35.8 | 1.0 | 0.7 |
| 6.4% Pt/SiO2 + CsPW (0.18) | 92.5 | 2.3 | 52.9 | 20.4 | 23.4 | 2.6 | 0.7 |
| 1% Pt/Al2O3 + CsPW (0.065) | 6.2 | 0.42 | 16.8 | 10.9 | 64.5 | 3.4 | 4.4 |
Although Pt plays the key role, the properties of acid co-catalysts (the strength and the number of acid sites) can also have a significant effect on the performance of bifunctional metal-acid catalysts. Therefore, several Brønsted solid acids were tested for comparison with CsPW. These include silica-supported heteropoly acid HPW and zeolites HZSM-5 with different Si/Al atomic ratios listed in Table S1 (ESI†), namely, 25%HPW/SiO2, HZSM-5-12 and HZSM-5-47. Their acid strength and proton site density are given in Table S2 (ESI†). Table 3 shows the effect of acid co-catalysts on the HDO of DMF at 100 °C. As seen, with all co-catalysts studied, full conversion of DMF was achieved, but the selectivity to hexane significantly depended on the acid strength. The strongest acids, CsPW and HPW/SiO2, gave >99% selectivity to hexane, whereas the weaker acids, HZSM-5 zeolites, gave 69–85% selectivity at 100 °C. HZSM-5-12 was notably more selective to hexane than HZSM-5-47, which can be explained by almost 4-fold higher H+ density in HZSM-5-12 (Table S2, ESI†). The deoxygenation activity of Pt–zeolite catalysts increased with increasing reaction temperature reaching ∼100% hexane yield at 120 °C. These results show that the HDO activity of the bifunctional catalysts correlates with the strength and the number of their acid sites.
| Catalyst | Temperature (°C) | Conversionb (%) | Product selectivityb (% mol) | ||||
|---|---|---|---|---|---|---|---|
| Hexane | DMTHF | 2-Hexanone | 2-Hexanol | Other | |||
a 1.6 kPa DMF, 20 ml min−1 H2 flow rate, 4 h TOS, 0.20 g catalyst 9.6% Pt/C + solid acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 w/w, 20 mg Pt/C, 1% Pt loading).
b Average conversion and product selectivity over 4 h TOS. 9 w/w, 20 mg Pt/C, 1% Pt loading).
b Average conversion and product selectivity over 4 h TOS.
|
|||||||
| Pt/C + CsPW | 100 | >99 | 99.9 | 0.1 | 0.0 | 0.0 | 0.0 |
| Pt/C + 25%HPW/SiO2 | 100 | >99 | 99.3 | 0.0 | 0.1 | 0.4 | 0.2 |
| Pt/C + HZMS-5-47 | 100 | >99 | 68.5 | 8.0 | 0.7 | 20.3 | 1.6 |
| Pt/C + HZMS-5-47 | 120 | >99 | 99.6 | 0.0 | 0.1 | 0.1 | 0.2 |
| Pt/C + HZMS-5-12 | 100 | >99 | 84.8 | 5.4 | 0.2 | 7.1 | 1.9 |
| Pt/C + HZMS-5-12 | 120 | >99 | 99.2 | 0.0 | 0.1 | 0.3 | 0.4 |
It is interesting to compare the HDO in the gas-phase system with that in the liquid phase. The results for the liquid-phase HDO of DMF over Pt/C + CsPW are given in Table S3 (ESI†).20 Full DMF conversion was achieved in 2 h reaction time, yielding mainly 2-hexanone and DMTHF. The selectivity to hexane was much lower than in the gas-phase (Table 1). It increased from 5 to 48% with increasing the temperature from 90 to 140 °C. Therefore, the liquid-phase HDO in a batch reactor is much less efficient and occurs under harsher conditions than the gas-phase process in a flow reactor. The reasons for the higher efficiency of the gas-phase process could be clarified via a more detailed kinetic study.
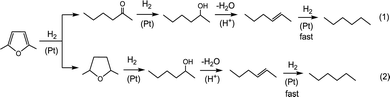
From the above data, the hydrodeoxygenation of DMF over Pt–CsPW can be represented by Scheme 1, which includes two parallel pathways (1 and 2) starting from interaction of DMF with Pt sites leading to DMF ring hydrogenolysis (pathway (1)) and ring hydrogenation (pathway (2)). The first reaction order in Pt/C (Fig. S2, ESI†) suggests that the initial steps catalysed by Pt are the rate-limiting steps in pathways (1) and (2), probably the formation of 2-hexanol. Furthermore, each pathway involves the dehydration of 2-hexanol to hexene on the proton sites as an effective driving force for the HDO reaction to occur. The final step of hexene-to-hexane hydrogenation on Pt sites is probably fast as no hexene was observed among the reaction products.
To support this reaction network, additional tests were carried out by passing 2-hexanone, 2-hexanol and 2,5-dimethyltetrahydrofuran (DMTHF) over CsPW, 9.6% Pt/C and 9.6% Pt/C + CsPW under conditions comparable to those in Table 1 (90 °C, 2 kPa substrate partial pressure, 20 ml min−1 H2 flow rate, 1% Pt loading). The reaction of 2-hexanone over Pt/C gave 91% selectivity to 2-hexanol at 87% conversion; only 6% of hexane was formed. Complete deoxygenation of ketones to alkanes on 10% Pt/C has been reported to occur at >300 °C.11,12 When 2-hexanone was passed over CsPW in the absence of Pt/C, practically no reaction was observed, whereas 100% hexane yield was obtained over Pt–CsPW in agreement with a previous report.12 The reaction of 2-hexanol over Pt/C gave only hexane, but at a very small alcohol conversion (5%). Interaction of 2-hexanol with CsPW in H2 or N2 yielded a mixture of 1-hexene and 2-hexene (cis and trans isomers) at >99% alcohol conversion. The reaction of 2-hexanol over Pt–CsPW in H2 produced n-hexane in >99 yield as expected. DMTHF over CsPW alone gave mainly cracking products at 2% DMTHF conversion. The monofunctional Pt/C catalyst in the absence of CsPW had a very low activity in DMTHF hydrogenolysis to form 2-hexanol in 88% selectivity at 1.6% DMTHF conversion, with only 8% of hexane being formed at 90 °C. Bifunctional Pt/C + CsPW catalysts had a good activity in HDO of DMTHF to give >99% hexane selectivity at 44% DMTHF conversion at 90 °C and 71% conversion at 100 °C. These tests confirm the hydrogenation–dehydration–hydrogenation sequence of reaction steps in Scheme 1 and demonstrate the importance of both Pt and H+ sites for the HDO reaction. Previous reports2,6,8 as well as this study show that the initial hydroconversion of DMF on Pt sites strongly favours the ring opening over the ring saturation, with the molar ratio of the primary reaction products [2-hexanone]/[DMTHF] ≈ 10. Moreover, the turnover rate of DMF hydroconversion in the gas phase on monofunctional Pt/C is 2400 times greater than that of DMTHF at 80 °C and 1 bar H2.8 This indicates that reaction pathway (1) should dominate by far over pathway (2) (Scheme 1).
In conclusion, Pt–CsPW catalyst deoxygenates DMF to n-hexane with 100% yield under very mild conditions (90 °C, 1 bar H2 pressure) in the gas phase. Mild reaction conditions exclude n-hexane isomerisation allowing complete conversion of DMF to alkane without carbon backbone alteration. The bifunctional Pt–CsPW catalyst is much more efficient than monofunctional Pt catalysts operating under harsh conditions.4,5,7 The gas-phase HDO in a flow system over Pt–CsPW is more efficient than the corresponding liquid-phase batch reaction. The proposed reaction network for the HDO of DMF includes a sequence of hydrogenolysis, hydrogenation and dehydration steps catalysed by Pt and H+ sites in a bifunctional catalyst. Combined action of metal and acid sites is essential for the effectiveness of this process. Facile dehydration of secondary alcohol intermediate, 2-hexanol, on proton sites is an effective driving force of the HDO process by bifunctional metal-acid catalysis.
We thank the Deanship of Scientific Research at King Faisal University, Saudi Arabia for the financial support under Nasher Track (Grant No. 206004).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS; (b) Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef; (c) R. M. West, Z. Y. Liu, M. Peter and J. A. Dumesic, ChemSusChem, 2008, 1, 417–424 CrossRef CAS; (d) G. W. Huber, J. N. Chheda, C. J. Barrett and J. A. Dumesic, Science, 2005, 308, 1446–1450 CrossRef CAS; (e) Z. J. Brentzel, K. J. Barnett, K. Huang, C. T. Maravelias, J. A. Dumesic and G. W. Huber, ChemSusChem, 2017, 10, 1351–1355 CrossRef CAS; (f) J. Kang, A. Vonderheide and V. V. Guliants, ChemSusChem, 2015, 8, 3044–3047 CrossRef CAS; (g) R. C. Runnebaum, T. Nimmanwudipong, J. Doan, D. E. Block and B. C. Gates, Catal. Lett., 2012, 142, 664–666 CrossRef CAS; (h) J. Kang, X. Liang and V. V. Guliants, ChemCatChem, 2017, 9, 282–286 CrossRef CAS.
- Y. L. Louie, J. Tang, A. M. L. Hell and A. T. Bell, Appl. Catal., B, 2017, 202, 557–568 CrossRef CAS.
- V. Vorotnikov and D. G. Vlachos, J. Phys. Chem. C, 2015, 119, 10417–10426 CrossRef CAS.
- A. Corma, O. de la Torre, M. Renz and N. Villandier, Angew. Chem., Int. Ed., 2011, 50, 2375–2378 CrossRef CAS.
- J. Yang, S. Li, L. Zhang, X. Liu, J. Wang, X. Pan, N. Li, A. Wang, Y. Cong, X. Wang and T. Zhang, Appl. Catal., B, 2017, 201, 266–277 CrossRef CAS.
- H. Goto, A. Takagaki, R. Kikuchi and S. T. Oyama, Appl. Catal., A, 2017, 548, 122–127 CrossRef CAS.
- F. Xue, D. Ma, T. Tong, X. Liu, Y. Hu, Y. Guo and Y. Wang, ACS Sustainable Chem. Eng., 2018, 6, 13107–13113 CrossRef CAS.
- H. Althikrallah, C. Kunstmann-Olsen, E. F. Kozhevnikova and I. V. Kozhevnikov, Catalysts, 2020, 10, 1171, DOI:10.3390/catal10101171.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- M. A. Alotaibi, E. F. Kozhevnikova and I. V. Kozhevnikov, J. Catal., 2012, 293, 141–144 CrossRef CAS.
- M. A. Alotaibi, E. F. Kozhevnikova and I. V. Kozhevnikov, Chem. Commun., 2012, 48, 7194–7196 RSC.
- K. Alharbi, E. F. Kozhevnikova and I. V. Kozhevnikov, Appl. Catal., A, 2015, 504, 457–462 CrossRef CAS.
- S. Itagaki, N. Matsuhashi, K. Taniguchi, K. Yamaguchi and N. Mizuno, Chem. Lett., 2014, 43, 1086–1088 CrossRef CAS.
- K. Alharbi, W. Alharbi, E. F. Kozhevnikova and I. V. Kozhevnikov, ACS Catal., 2016, 6, 2067–2075 CrossRef CAS.
- T. Okuhara, N. Mizuno and M. Misono, Adv. Catal., 1996, 41, 113–252 CAS.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171–198 CrossRef CAS.
- M. A. Alotaibi, E. F. Kozhevnikova and I. V. Kozhevnikov, Appl. Catal., A, 2012, 447–448, 32–40 CrossRef CAS.
- A. Alazman, D. Belic, E. F. Kozhevnikova and I. V. Kozhevnikov, J. Catal., 2018, 357, 80–89 CrossRef.
- P. B. Weisz, Adv. Catal., 1962, 13, 137–190 CAS.
- The reaction was carried out in a stainless steel autoclave at 90–140 °C, 20 bar H2 pressure (RT) and 600 rpm stirring speed using decane as a solvent. Under such conditions, the reaction did not depend on the stirring speed, and hence was not limited by external diffusion.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and kinetic plots. See DOI: 10.1039/d0cc06934e |
| This journal is © The Royal Society of Chemistry 2021 |