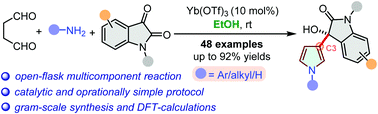
Direct catalytic synthesis of β-(C3)-substituted pyrroles: a complementary addition to the Paal–Knorr reaction†
Abstract
The synthesis of β-(C3)-functionalized pyrroles is a challenging task and requires a multistep protocol. An operationally simple direct catalytic synthesis of β-substituted pyrroles has been developed. This one-pot multicomponent method combined aqueous succinaldehyde as 1,4-dicarbonyl, primary amines, and isatins to access hydroxyl-oxindole β-tethered pyrroles. Direct synthesis of the β-substituted free NH-pyrrole is the central intensity of this work. DFT-calculations and preliminary mechanism investigation support the possible reaction pathway.


 Please wait while we load your content...
Please wait while we load your content...