Open Access Article
Open Access ArticleExperimental validation of pressure swing regeneration for faster cycling in sorption enhanced dimethyl ether synthesis†
Jasper
van Kampen
 *ab,
Saskia
Booneveld
a,
Jurriaan
Boon
*ab,
Saskia
Booneveld
a,
Jurriaan
Boon
 ab,
Jaap
Vente
a and
Martin
van Sint Annaland
ab,
Jaap
Vente
a and
Martin
van Sint Annaland
 b
b
aBiomass and Energy Efficiency, TNO Energy Transition, P. O. Box 15, 1755 ZG Petten, The Netherlands. E-mail: jasper.vankampen@tno.nl
bChemical Process Intensification, TU/e, P. O. Box 513, 5600 MB Eindhoven, The Netherlands. E-mail: j.v.kampen@tue.nl
First published on 2nd October 2020
Abstract
Sorption enhanced dimethyl ether synthesis (SEDMES) is a novel DME production route from CO2-rich feedstocks. In situ water removal by adsorption results in high single-pass conversions, thereby circumventing the disadvantages of conventional routes, such as low carbon efficiency, energy intensive downstream separation and large recycling. The first-time demonstration of pressure swing regeneration with 80% single-pass carbon selectivity to DME allows for an enormous increase in productivity. Already a factor four increase compared to temperature swing regeneration is achieved, unlocking the potential of SEDMES as a carbon utilisation technology.
Dimethyl ether (DME) is a valuable platform chemical and synthetic fuel. It is expected to play an important role in the current energy transition, where fossil-based fuels and chemicals have to be replaced by products from renewable feedstocks.1–3 This is envisioned by the chemical recycling of carbon dioxide directly,4 as well as by indirect conversion via biomass-based, CO2-rich syngas. However, the production of DME from a CO2-rich feedstock or CO2 directly via conventional processes is limited and therefore considered unattractive.5,6 Similar to many other industrial CO2 utilisation processes, the main hurdle is the production and efficient handling of steam.4,7,8 Steam separation enhancement is a promising route for CO2 utilisation.7 The concept of separation enhancement is based on Le Chatelier's principle, according to which an equilibrium limited reaction is shifted to enhance conversion by selectively removing reaction products, and is mainly utilised for various processes and products considering CO2 separation.9,10 Sorption enhanced DME synthesis (SEDMES), which combines direct DME synthesis with in situ water removal using a solid adsorbent, is a promising process intensification for the direct production of DME from CO2.7,11–13 While enabling increased single-pass conversion and selectivity, experimental studies have indicated that time and energy intensive temperature swing regeneration would be required for adsorbent regeneration.7,13 A potentially faster and more energy efficient approach is pressure swing regeneration as recently indicated in a SEDMES modelling study.13 The current communication reports the results of an experimental investigation on the bench-scale sorption enhanced production of DME. Special attention is being paid to the mode of regeneration, as the key to an economically attractive process.
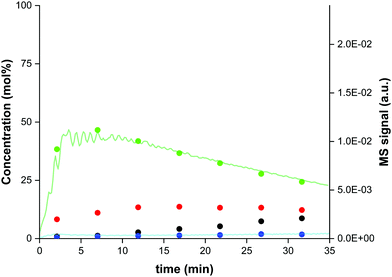
Sorption enhanced DME synthesis is validated experimentally using a 2 litre bench-scale reactor, details of which are provided in the ESI.†Fig. 1 shows a representative breakthrough experiment of sorption enhanced DME synthesis. CO2-rich syngas (CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO = 2
CO = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
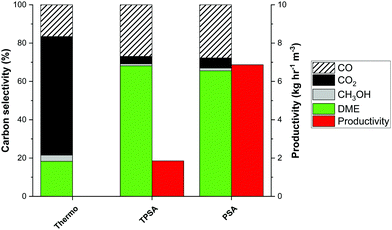
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, stoichiometric H2), as typical for biomass-derived syngas, is fed to a previously regenerated column, at 250–275 °C and 25 bar, containing a homogeneous mixture of a CuZnAl catalyst, a γ-Al2O3 catalyst, and a 3A zeolite adsorbent, all of which are commercially available. The reactor outlet composition is measured using mass spectrometry and gas chromatography, as shown in Fig. 1. Prior to steam breakthrough, DME and unconverted CO are the primary products. After steam breakthrough, the concentration of DME drops, accompanied by the breakthrough of CO2 and methanol, indicating saturation of the adsorbent. These bench-scale test results demonstrate high performance with over 80% integral carbon selectivity towards DME when using pressure swing regeneration, without the need for a temperature swing, for the first time in the open literature. In Fig. 2 the experimental carbon distribution for SEDMES at 275 °C is shown, indicating the single-pass conversion increase from 18% for conventional synthesis (represented as the thermodynamic equilibrium) to 68%. Crucially, the experiment serves to show how a similar conversion and selectivity can be obtained with pressure swing regeneration (PSA) in comparison with previously reported experiments with a combined temperature and pressure swing regeneration (TPSA). As no time is required for heating and cooling, the adsorbent regeneration time can be reduced from 360 minutes to 60 minutes under the current conditions (Fig. 2). As a result, the DME productivity is already increased by a factor of four, with further optimisation possible.
1, stoichiometric H2), as typical for biomass-derived syngas, is fed to a previously regenerated column, at 250–275 °C and 25 bar, containing a homogeneous mixture of a CuZnAl catalyst, a γ-Al2O3 catalyst, and a 3A zeolite adsorbent, all of which are commercially available. The reactor outlet composition is measured using mass spectrometry and gas chromatography, as shown in Fig. 1. Prior to steam breakthrough, DME and unconverted CO are the primary products. After steam breakthrough, the concentration of DME drops, accompanied by the breakthrough of CO2 and methanol, indicating saturation of the adsorbent. These bench-scale test results demonstrate high performance with over 80% integral carbon selectivity towards DME when using pressure swing regeneration, without the need for a temperature swing, for the first time in the open literature. In Fig. 2 the experimental carbon distribution for SEDMES at 275 °C is shown, indicating the single-pass conversion increase from 18% for conventional synthesis (represented as the thermodynamic equilibrium) to 68%. Crucially, the experiment serves to show how a similar conversion and selectivity can be obtained with pressure swing regeneration (PSA) in comparison with previously reported experiments with a combined temperature and pressure swing regeneration (TPSA). As no time is required for heating and cooling, the adsorbent regeneration time can be reduced from 360 minutes to 60 minutes under the current conditions (Fig. 2). As a result, the DME productivity is already increased by a factor of four, with further optimisation possible.
Following the proof of feasibility of pressure swing regeneration, the cycle design of the sorption enhanced process becomes essential. The combination of the adsorption duration (ADS) and regeneration time (DES) allows optimising the trade-off for DME selectivity and productivity, which is shown in Fig. 3. Shorter adsorption times potentially result in an increased production rate. The larger reactor column requirement (m3) when the purge time does not decrease with the adsorption time, however, results in a drop in cyclic productivity (kg h−1 m−3). Considering the minimum number of columns required for any given adsorption and purge time, the productivity could be significantly boosted for shorter cycle times with the highest ADS/DES ratio (red bars in Fig. 3). The productivities reported in Fig. 3 correspond to 0.04–0.06 kg h−1 kgcat−1. This is a major improvement compared to the previously reported TPSA cycle and close to direct DME pilot plant productivity for CO to DME, which would strongly deteriorate for CO2-rich feed.13
This increase in productivity shows the impact of the demonstrated PSA regeneration on the SEDMES process performance. Benefiting from this work, optimisation of the cycle design by means of modelling and experimental validation should further unlock the potential of SEDMES as an efficient carbon utilisation technology. Followed by the techno-economic and life cycle analyses, the economic and carbon mitigating benefits of SEDMES over conventional DME synthesis technology can also now be addressed.
This work has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement no. 727600.
Conflicts of interest
There are no conflicts to declare.References
- T. A. Semelsberger, R. L. Borup and H. L. Greene, Dimethyl ether (DME) as an alternative fuel, J. Power Sources, 2006, 156, 497–511 CrossRef CAS.
- C. Arcoumanis, C. Bae, R. Crookes and E. Kinoshita, The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: a review, Fuel, 2008, 87, 1014–1030 CrossRef CAS.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether, J. Org. Chem., 2009, 74, 487–498 CrossRef CAS.
- G. Centi and S. Perathoner, Opportunities and prospects in the chemical recycling of carbon dioxide to fuels, Catal. Today, 2009, 148, 191–205 CrossRef CAS.
- V. Dieterich, A. Buttler, A. Hanel, H. Spliethoff and S. Fendt, Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: a review, Energy Environ. Sci., 2020 10.1039/d0ee01187h.
- R. J. Detz, J. N. H. Reek and B. C. C. van der Zwaan, The future of solar fuels: when could they become competitive?, Energy Environ. Sci., 2018, 11, 1653–1669 RSC.
- J. van Kampen, J. Boon, F. van Berkel, J. Vente and M. van Sint Annaland, Steam separation enhanced reactions: Review and outlook, Chem. Eng. J., 2019, 374, 1286–1303 CrossRef CAS.
- A. Katelhon, R. Meys, S. Deutz, S. Suh and A. Bardow, Climate change mitigation potential of carbon capture and utilization in the chemical industry, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef CAS.
- A. E. Rodrigues, L. M. Madeira, Y.-J. Wu and R. Faria, Sorption Enhanced Reaction Processes, Sustainable Chemistry Series, World Scientific, 2017, vol. 1, DOI:10.1142/q0103.
- J. C. Abanades, J. Boon, P. Cobden, K. Coenen, D. S. M. Constantino, R. P. V. Faria, J. R. Fernández, F. Gallucci, M. C. Iliuta, A. E. Rodrigues, E. van Dijk and M. van Sint Annaland, Sorption Enhancement of Chemical Processes, Academic Press, 1st edn, 2017 Search PubMed.
- I. Iliuta, M. C. Iliuta and F. Larachi, Sorption-enhanced dimethyl ether synthesis—multiscale reactor modeling, Chem. Eng. Sci., 2011, 66, 2241–2251 CrossRef CAS.
- J. Boon, J. van Kampen, R. Hoogendoorn, S. Tanase, F. P. F. van Berkel and M. van Sint Annaland, Reversible deactivation of γ-alumina by steam in the gas-phase dehydration of methanol to dimethyl ether, Catal. Commun., 2019, 119, 22–27 CrossRef CAS.
- J. van Kampen, J. Boon, J. Vente and M. van Sint Annaland, Sorption enhanced dimethyl ether synthesis for high efficiency carbon conversion: modelling and cycle design, J. CO2 Util., 2020, 37, 295–308 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc06093c |
| This journal is © The Royal Society of Chemistry 2020 |