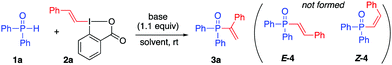Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transition metal-free and regioselective vinylation of phosphine oxides and H-phosphinates with VBX reagents†
Laura
Castoldi‡
 a,
Adam A.
Rajkiewicz‡
a,
Adam A.
Rajkiewicz‡
 ab and
Berit
Olofsson
ab and
Berit
Olofsson
 *a
*a
aDepartment of Organic Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91 Stockholm, Sweden. E-mail: berit.olofsson@su.se
bCentre of New Technologies, University of Warsaw, Banacha 2C, 02-097, Warsaw, Poland
First published on 27th October 2020
Abstract
A series of phosphine oxides and H-phosphinates were vinylated in the presence of the iodine(III) reagents vinylbenziodoxolones (VBX), providing the corresponding alk-1-enyl phosphine oxides and alk-1-enyl phosphinates in good yields with complete chemo- and regioselectivity. The vinylation proceeds in open flask under mild and transition metal-free conditions.
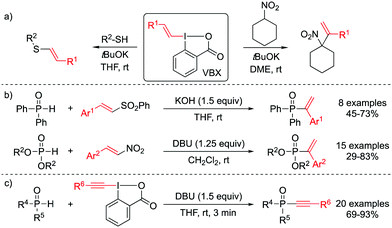
Among the plethora of iodine(III) reagents,1 benziodoxolone scaffolds have been introduced as more stable alternatives to iodonium salts in alkynylation and trifluoromethylation reactions.2 Vinylbenzoiodoxolones (VBX) were recently introduced as efficient vinylating agents.3 They have since been used in metal-catalyzed cross couplings and C–H vinylations,4 as well as in several elegant metal-free reactions.4c,e,5 We have demonstrated the unique reactivity of VBX in the vinylation of nitrocyclohexane, with regioselective formation of the terminal alkene (Scheme 1a), whereas vinyliodonium salts gave the internal alkene.3 To the contrary, the VBX vinylation of thiols and mercaptothiazoles resulted in internal alkene products with high E-selectivity (Scheme 1a),6 while vinyliodonium salts give the Z-isomer through a vinylic SN2 mechanism.7
The synthesis of VBX reagents is straightforward, and can either be performed in a one-pot reaction from 2-iodobenzoic acid,3 or via a preformed iodine(III) species that is reacted with a suitable vinylboronic acid.4d,g Heteroatom nucleophiles can be added to EBX reagents to further increase the scope of VBX reagents.4f,g,8
Alkenylphosphorus compounds are primary building blocks in a series of transformations, including reduction,9 epoxidation,10 Michael addition11 and cycloaddition.12 Moreover, vinylated phosphorus compounds have become of interest for the development of pharmacologically important and biologically active compounds such as antibiotics13 and enzyme inhibitors.14 Alkenyl phosphine oxides and phosphonates are also valuable monomers in polymer and materials chemistry.15
Traditionally, the synthesis of alkenyl phosphorus compounds relies on transition metal-catalyzed cross coupling methodologies;16 and terminal alkenes can be prepared through metal-catalyzed hydrophosphorylation of alkynes.17 Despite the utility of these methods, common drawbacks include the removal of the transition metal from the final product, the toxicity and the cost of the catalyst, and need for preparation of non-commercial ligands.18 Metal-free methodologies for P–C bond formation are hence highly desirable.19 While several efficient methods for vinylation of phosphine oxides to provide the internal alkene product have recently been reported,20 the synthesis of the corresponding terminal alkene product remains difficult. Methods utilizing vinyl sulfones or nitro olefins as vinylation reagent have only been demonstrated with a very limited phosphorus substrate scope (Scheme 1b).21
Vinyliodonium salts have been employed in a mild copper-catalyzed procedure with H-phosphonates to afford (E) 2-arylvinylphosphonates.22 In 2014, the benziodoxolone reagent EBX was demonstrated to be efficient in metal-free alkynylation of H-phosphites, H-phosphinates and secondary phosphine oxides under mild conditions and short reaction times (Scheme 1c).23 We envisaged that a method involving the novel VBX reagent could be attractive for the construction of C–P bonds. Herein, we report a mild and metal-free, open flask methodology to vinylate phosphine oxides and phosphinates with complete regioselectivity towards the terminal alkene.
Diphenylphosphine oxide (1a) was chosen as the model substrate to study the vinylation of phosphorous compounds with VBX 2a. Surprisingly, the vinylation proceeded with complete regioselectivity in favor of the terminal alkene 3a without formation of any internal alkenes 4 (Table 1).24 A series of organic and inorganic bases were screened in THF at room temperature (entries 1–6). The use of DBU and BTMG proved superior to other bases (entries 5 and 6), while no reactivity was detected in the absence of base (entry 4). DBU was selected for further investigations as BTMG is more expensive, and THF was found to be the best solvent (entries 5 and 7–9). Shortening the reaction time to 1 h and reduced amount of VBX resulted in small drops in yield (entries 10 and 11). Pleasingly, 3a was obtained in 94% isolated yield by lowering the concentration of the reaction to 0.05 M, likely due to increased solubility of VBX (entry 13). We have previously demonstrated that the formed iodobenzoic acid can be recovered and reused for VBX synthesis, thus increasing the sustainability of the process.6
| Entry | Base | Solvent | Conc. [mol L−1] | Time [h] | Yield 3ab [%] |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.05 mmol) and base were stirred in solvent for 5 min before addition of 2a (1.2 equiv.). b 1H-NMR yield using trimethoxybenzene as internal standard, isolated yield in brackets. c At 60 °C. d 1.0 equiv. of 2a. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; BTMG = 2-tert-butyl-1,1,3,3-tetramethylguanidine; DMAP = 4-(dimethylamino)pyridine. | |||||
| 1 | Et3N | THF | 0.1 | 20 | 5 |
| 2 | DMAP | THF | 0.1 | 20 | <5 |
| 3c | Cs2CO3 | THF | 0.1 | 20 | 62 |
| 4 | — | THF | 0.1 | 20 | 0 |
| 5 | DBU | THF | 0.1 | 20 | 88 |
| 6 | BTMG | THF | 0.1 | 20 | 90 |
| 7 | DBU | Et2O | 0.1 | 20 | 40 |
| 8 | DBU | Toluene | 0.1 | 20 | 64 |
| 9 | DBU | EtOAc | 0.1 | 20 | 76 |
| 10 | DBU | THF | 0.1 | 1 | 84 |
| 11d | DBU | THF | 0.1 | 1 | 80 |
| 12 | DBU | THF | 0.25 | 1 | 78 |
| 13 | DBU | THF | 0.05 | 1 | 93 (94) |
With the optimized conditions established, the substrate scope was examined with symmetric and unsymmetric diarylphosphine oxides 1 (Scheme 2a and b). Substrates with electron donating substituents provided high yields of the desired product (3a–g), including the sterically congested products 3d and 3e. A fluorine substituent was also tolerated (3h), whereas electron withdrawing substituents drastically reduced the reactivity. A modest yield of 3i was obtained under standard conditions, which could be improved to 27% yield by increasing the temperature and prolonging the reaction time.
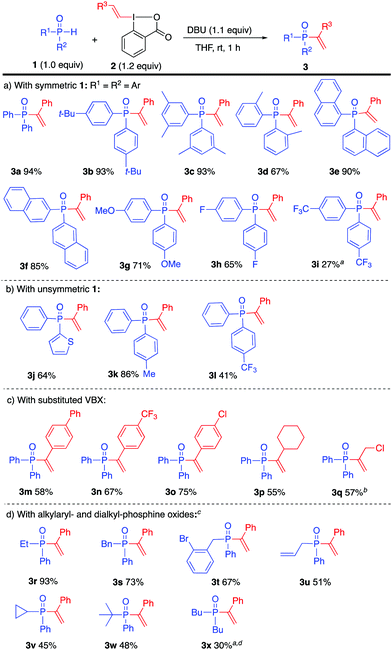 | ||
Scheme 2 Scope with diaryl- and alkylaryl-phosphine oxides. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At 60 °C, 20 h. b At 60 °C, 20 h. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At –10 °C, 2 h. c At –10 °C, 2 h. c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At 60 °C, 2 h. d At 60 °C, 2 h. d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NMR yield. NMR yield. | ||
Unsymmetric phosphine oxides were prepared from alkyl phenyl-H-phosphinates by reaction with the corresponding Grignard/organolithium reagent,24,25 and subsequently examined in the vinylation. The unsymmetric product 3j, bearing a thiophene moiety, and phenyl(p-tolyl)phosphine oxide (3k) were obtained in good to high yields. The presence of a CF3 group in product 3l decreased the yield to 41%.
The scope of VBX reagents was investigated by vinylation of diphenylphosphine oxide (1a, Scheme 2c). VBX reagents with both electron-donating and -withdrawing substituents on the styryl moiety were well tolerated and gave the desired alk-1-enyl phosphine oxides 3m–o in good yields. Aliphatic VBX reagents have previously proved problematic and provided complex product mixtures.3,6 Pleasingly, aliphatic VBX reagents could be employed in the P-vinylation to provide the cyclohexyl product 3p. Also the 3-chloropropenyl product 3q, with a sensitive functional group, was successfully synthesized upon lowering the temperature. However, the Z-configured sulfonamide-substituted VBX reported by Waser4c and our core-dimethylated Z-VBX derivative6 did not afford the desired product. Trisubstituted VBXs also proved unreactive.24
Alkylarylphosphine oxides proved to be less reactive towards VBX, and required additional optimization. Ethyl-phenylphosphine oxide could be vinylated to give 3r in excellent yield within 2 hours at 60 °C (Scheme 2d). Other alkyl- and allyl derivatives were vinylated in the same manner to afford products 3s–v. The successful synthesis of tert-butyl product 3w illustrates the high tolerance for sterical hindrance. Dialkyl-phosphine oxides were, on the other hand very unreactive, and product 3x could only be obtained in modest yield upon prolonged reaction time at 60 °C.
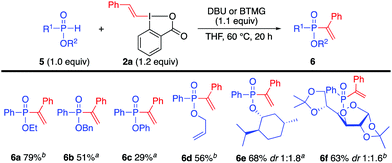
We have recently investigated the effect of several core-substituted VBX reagents in the vinylation of thiols,6 and substituent effects have previously been demonstrated with EBX reagents26 and other iodine(III) compounds.27 We hence investigated the influence of core-substituted VBX reagents in P-vinylation of H-phosphinates, as they proved less reactive than phosphine oxides. Phosphinate 5a was reacted with a series of core-substituted VBX reagents to investigate electronic and steric effects (see ESI†). Me2-VBX, which we previously reported to improve the S-vinylation yields,6 showed comparable reactivity to the unsubstituted VBX, whereas other changes in the core structure reduced the yield of 6a. A control reaction with the corresponding acyclic vinyliodonium salt gave only 10% yield of 6a.
The desired alkenylation of H-phosphinates proved viable at 60 °C for 20 h using either DBU or BTMG,24 and the scope was examined with both bases as the outcome was substrate dependent (Scheme 3).24 Ethyl phenylphosphinate and benzyl phenylphosphinate gave 6a and 6b, respectively. Phenoxy and allyloxy products 6c and 6d could also be obtained in this fashion (the isolated yield of 6c is affected by the instability of this compound). More complex substrates were also suitable for this methodology, as exemplified by the vinylation of (R)-phenyl menthoxy phosphinate 5e and glucose diacetonide-derived H-phosphonate 5f to give products 6e and 6f in good yields as a mixture of diastereoisomers. The vinylation of H-phosphonates proved to be sluggish, despite optimization attempts under various conditions.24
The different regiochemistry obtained in vinylations of phosphorus and sulfur nucleophiles with VBX is intriguing, and preliminary tests with radical scavengers were conducted to investigate whether a radical pathway could be operating. The reaction of diphenylphosphine oxide (1a) with TEMPO under standard conditions afforded 82% yield of 3a, which is comparable with the results without additive, indicating that a radical pathway is unlikely.24 The observed regiochemistry would be consistent with a phospha-Michael-type addition28 followed by proton shift and elimination, but alternative pathways cannot be excluded at this point.
To conclude, the recently discovered reagent VBX was employed to efficiently vinylate a wide range of phosphine oxides and H-phosphinates. The reactions were performed in open flask under mild and transition metal free conditions, and delicate functional groups were tolerated. Both aromatic and aliphatic vinyl moieties could be transferred, and the electronic and steric influences of core-substituted VBX derivatives were investigated. We are currently performing an extensive mechanistic investigation on the reactivity of VBX with several nucleophiles under metal-free conditions, and will report the results in due time.
We thank Ester Maria Di Tommaso for analytical support. We acknowledge Carl Trygger Foundation (CTS 17:341, Fellowship for LC), the National Science Centre Poland (2016/22/E/ST5/00566) and The Warsaw University's Integrated Development Programme (both Fellowships for AAR), and the Swedish Research Council (2015-04404, 2019-04232) for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) V. V. Zhdankin, Hypervalent Iodine Chemistry, Wiley, Chichester, UK, 2014 Search PubMed; (b) Hypervalent Iodine Chemistry, ed. T. Wirth, Springer International Publishing, Cham, 2016 Search PubMed; (c) The Chemistry of Hypervalent Halogen Compounds, ed. B. Olofsson, I. Marek and Z. Rappoport, Wiley, 2019 Search PubMed.
- (a) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650–682 CrossRef CAS; (b) Y. Li, D. P. Hari, M. V. Vita and J. Waser, Angew. Chem., Int. Ed., 2016, 55, 4436–4454 CrossRef CAS; (c) D. P. Hari, P. Caramenti and J. Waser, Acc. Chem. Res., 2018, 51, 3212–3225 CrossRef CAS.
- E. Stridfeldt, A. Seemann, M. J. Bouma, C. Dey, A. Ertan and B. Olofsson, Chem. – Eur. J., 2016, 22, 16066–16070 CrossRef CAS.
- (a) J. Wu, X. Deng, H. Hirao and N. Yoshikai, J. Am. Chem. Soc., 2016, 138, 9105–9108 CrossRef CAS; (b) J. Wu, K. Xu, H. Hirao and N. Yoshikai, Chem. – Eur. J., 2017, 23, 1521–1525 CrossRef CAS; (c) P. Caramenti, N. Declas, R. Tessier, M. D. Wodrich and J. Waser, Chem. Sci., 2019, 10, 3223–3230 RSC; (d) A. Boelke, L. D. Caspers and B. J. Nachtsheim, Org. Lett., 2017, 19, 5344–5347 CrossRef CAS; (e) B. Liu, C.-H. Lim and G. M. Miyake, J. Am. Chem. Soc., 2018, 140, 12829–12835 CrossRef CAS; (f) R. Tessier, J. Ceballos, N. Guidotti, R. Simonet-Davin, B. Fierz and J. Waser, Chem, 2019, 5, 2243–2263 CrossRef CAS; (g) G. Pisella, A. Gagnebin and J. Waser, Org. Lett., 2020, 22, 3884–3889 CrossRef CAS.
- (a) J. Davies, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2017, 56, 13361–13365 CrossRef CAS; (b) S. G. E. Amos, S. Nicolai, A. Gagnebin, F. L. Vaillant and J. Waser, J. Org. Chem., 2019, 84, 3687–3701 CrossRef CAS; (c) B. Liu, J. Alegre-Requena, R. Paton and G. Miyake, Chem. – Eur. J., 2020, 26, 2386–2394 CrossRef CAS; (d) N. Declas and J. Waser, Angew. Chem., Int. Ed., 2020, 59, 18256–18260 CrossRef CAS.
- L. Castoldi, E. M. Di Tommaso, M. Reitti, B. Gräfen and B. Olofsson, Angew. Chem., Int. Ed., 2020, 59, 15512–15516 CrossRef CAS.
- (a) M. Ochiai, S. Yamamoto, T. Suefuji and D.-W. Chen, Org. Lett., 2001, 3, 2753–2756 CrossRef CAS ; For recent developments with vinyliodonium and vinylchloronium salts, see: ; (b) Á. Mészáros, A. Székely, A. Stirling and Z. Novák, Angew. Chem., Int. Ed., 2018, 57, 6643–6647 CrossRef; (c) Y. Watanabe, T. Takagi, K. Miyamoto, J. Kanazawa and M. Uchiyama, Org. Lett., 2020, 22, 3469–3473 CrossRef CAS.
- (a) B. Wu, J. Wu and N. Yoshikai, Chem. – Asian J., 2017, 12, 3123–3127 CrossRef CAS; (b) D. Shimbo, A. Shibata, M. Yudasaka, T. Maruyama, N. Tada, B. Uno and A. Itoh, Org. Lett., 2019, 21, 9769–9773 CrossRef CAS; (c) J. Wu, X. Deng and N. Yoshikai, Chem. – Eur. J., 2019, 25, 7839–7842 CrossRef CAS; (d) P. Caramenti, N. Declas, R. Tessier, M. D. Wodrich and J. Waser, Chem. Sci., 2019, 10, 3223–3230 RSC.
- (a) P. Cheruku, A. Paptchikhine, T. L. Church and P. G. Andersson, J. Am. Chem. Soc., 2009, 131, 8285–8289 CrossRef CAS; (b) K. Dong, Z. Wang and K. Ding, J. Am. Chem. Soc., 2012, 134, 12474–12477 CrossRef CAS; (c) D.-Y. Wang, X.-P. Hu, J. Deng, S.-B. Yu, Z.-C. Duan and Z. Zheng, J. Org. Chem., 2009, 74, 4408–4410 CrossRef CAS.
- Y. Ono and L.-B. Han, Tetrahedron Lett., 2006, 47, 421–424 CrossRef CAS.
- S. Sulzer-Mossé, M. Tissot and A. Alexakis, Org. Lett., 2007, 9, 3749–3752 CrossRef.
- N. Rabasso and A. Fadel, Tetrahedron Lett., 2010, 51, 60–63 CrossRef CAS.
- C. Giordano and G. Castaldi, J. Org. Chem., 1989, 54, 1470–1473 CrossRef CAS.
- (a) J.-J. Shie, J.-M. Fang, S.-Y. Wang, K.-C. Tsai, Y.-S. E. Cheng, A.-S. Yang, S.-C. Hsiao, C.-Y. Su and C.-H. Wong, J. Am. Chem. Soc., 2007, 129, 11892–11893 CrossRef CAS; (b) X. Lu, C. Sun, W. J. Valentine, S. E, J. Liu, G. Tigyi and R. Bittman, J. Org. Chem., 2009, 74, 3192–3195 CrossRef CAS; (c) Z. Liu, N. MacRitchie, S. Pyne, N. J. Pyne and R. Bittman, Bioorg. Med. Chem., 2013, 21, 2503–2510 CrossRef CAS.
- (a) M. Schmider, E. Müh, J. E. Klee and R. Mülhaupt, Macromolecules, 2005, 38, 9548–9555 CrossRef CAS; (b) J. Parvole and P. Jannasch, Macromolecules, 2008, 41, 3893–3903 CrossRef CAS; (c) T. Sato, M. Hasegawa, M. Seno and T. Hirano, J. Appl. Polym. Sci., 2008, 109, 3746–3752 CrossRef CAS; (d) S. V. Levchik and E. D. Weil, Polym. Int., 2005, 54, 11–35 CrossRef CAS.
- (a) L. Liu, Y. Wang, Z. Zeng, P. Xu, Y. Gao, Y. Yin and Y. Zhao, Adv. Synth. Catal., 2013, 355, 659–666 CrossRef CAS; (b) Y. Wu, L. Liu, K. Yan, P. Xu, Y. Gao and Y. Zhao, J. Org. Chem., 2014, 79, 8118–8127 CrossRef CAS; (c) J. Hu, N. Zhao, B. Yang, G. Wang, L.-N. Guo, Y.-M. Liang and S.-D. Yang, Chem. – Eur. J., 2011, 17, 5516–5521 CrossRef CAS; (d) G. Hu, Y. Gao and Y. Zhao, Org. Lett., 2014, 16, 4464–4467 CrossRef CAS; (e) M. Niu, H. Fu, Y. Jiang and Y. Zhao, Chem. Commun., 2007, 272–274 RSC; (f) G. Evano, K. Tadiparthi and F. Couty, Chem. Commun., 2011, 47, 179–181 RSC.
- (a) L.-B. Han and M. Tanaka, J. Am. Chem. Soc., 1996, 118, 1571–1572 CrossRef CAS; (b) Q. Xu, R. Shen, Y. Ono, R. Nagahata, S. Shimada, M. Goto and L.-B. Han, Chem. Commun., 2011, 47, 2333–2335 RSC; (c) N. Dobashi, K. Fuse, T. Hoshino, J. Kanada, T. Kashiwabara, C. Kobata, S. K. Nune and M. Tanaka, Tetrahedron Lett., 2007, 48, 4669–4673 CrossRef CAS; (d) L.-B. Han, C. Zhang, H. Yazawa and S. Shimada, J. Am. Chem. Soc., 2004, 126, 5080–5081 CrossRef CAS; (e) H. Li-Biao, O. Yutaka, X. Qing and S. Shigeru, Bull. Chem. Soc. Jpn., 2010, 83, 1086–1099 CrossRef.
- C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219–9280 CrossRef CAS.
- Recent metal-free examples for P–C bond formation: (a) B. Yang, S.-M. Hou, S.-Y. Ding, X.-N. Zhao, Y. Gao, X. Wang and S.-D. Yang, Adv. Synth. Catal., 2018, 360, 4470–4474 CrossRef CAS; (b) Q. Fu, Z.-Y. Bo, J.-H. Ye, T. Ju, H. Huang, L.-L. Liao and D.-G. Yu, Nat. Commun., 2019, 10, 3592 CrossRef.
- (a) L. Liu, D. Zhou, J. Dong, Y. Zhou, S.-F. Yin and L.-B. Han, J. Org. Chem., 2018, 83, 4190–4196 CrossRef CAS; (b) J. Shen, R.-X. Yu, Y. Luo, L.-X. Zhu, Y. Zhang, X. Wang, B. Xiao, J.-B. Cheng, B. Yang and G.-Z. Li, Eur. J. Org. Chem., 2019, 2065–2070 CrossRef CAS; (c) H. Wang, Y. Li, Z. Tang, S. Wang, H. Zhang, H. Cong and A. Lei, ACS Catal., 2018, 8, 10599–10605 CrossRef CAS.
- (a) H.-M. Guo, Q.-Q. Zhou, X. Jiang, D.-Q. Shi and W.-J. Xiao, Adv. Synth. Catal., 2017, 359, 4141–4146 CrossRef CAS; (b) N. Ono, N. Banshou, S. Ito, T. Murashima and T. Ogawa, J. Heterocycl. Chem., 1997, 34, 1243–1246 CrossRef CAS; (c) H.-X. Chen, L.-J. Huang, J.-B. Liu, J. Weng and G. Lu, Phosphorus, Sulfur Silicon Relat. Elem., 2014, 189, 1858–1866 CrossRef CAS.
- S. Thielges, P. Bisseret and J. Eustache, Org. Lett., 2005, 7, 681–684 CrossRef CAS.
- C. C. Chen and J. Waser, Chem. Commun., 2014, 50, 12923–12926 RSC.
- See the ESI,† for details.
- A. Tripolszky and G. Keglevich, Lett. Org. Chem., 2018, 15, 387–393 CrossRef CAS.
- (a) D. Fernández González, J. P. Brand, R. Mondière and J. Waser, Adv. Synth. Catal., 2013, 355, 1631–1639 CrossRef; (b) A. H. Abazid and B. J. Nachtsheim, Angew. Chem., Int. Ed., 2020, 59, 1479–1484 CrossRef CAS.
- (a) A.-A. Guilbault and C. Y. Legault, ACS Catal., 2012, 2, 219–222 CrossRef CAS; (b) J. Malmgren, S. Santoro, N. Jalalian, F. Himo and B. Olofsson, Chem. – Eur. J., 2013, 19, 10334–10342 CrossRef CAS.
- D. Enders, A. Saint-Dizier, M.-I. Lannou and A. Lenzen, Eur. J. Org. Chem., 2006, 29–49 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, analytical data and NMR spectra of novel compounds. See DOI: 10.1039/d0cc05992g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |