Ligand-free copper-catalyzed regio- and stereoselective 1,1-alkylmonofluoroalkylation of terminal alkynes†
Abstract
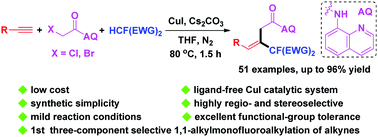
The copper-catalyzed highly regio- and stereoselective 1,1-alkylmonofluoroalkylation of terminal alkynes with α-chloroacetamides and dialkyl 2-fluoromalonate or 2-fluoro-N,N-dialkyl-3-oxobutanamide without an external ligand has been realized. With this novel methodology, (E)-β-monofluoroalkyl-β,γ-unsaturated amides containing quaternary C–F centers can be easily constructed in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...