Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emission color-tunable oxazol(in)yl-substituted excited-state intramolecular proton transfer (ESIPT)-based luminophores†
Dominik
Göbel‡
a,
Pascal
Rusch‡
bc,
Daniel
Duvinage
 d,
Nadja C.
Bigall
d,
Nadja C.
Bigall
 *bc and
Boris J.
Nachtsheim
*bc and
Boris J.
Nachtsheim
 *a
*a
aInstitute for Organic and Analytical Chemistry, University of Bremen, Leobener Straße NW2, D-28359 Bremen, Germany. E-mail: nachtsheim@uni-bremen.de
bLeibniz University Hannover, Institute of Physical Chemistry and Electrochemistry, Callinstraße 3a, D-30167 Hannover, Germany. E-mail: nadja.bigall@pci.uni-hannover.de
cCluster of Excellence PhoenixD (Photonics, Optics, and Engineering – Innovation Across Disciplines), Hannover, Germany
dInstitute for Inorganic and Crystallographic Chemistry, University of Bremen, Leobener Straße NW2, D-28359 Bremen, Germany
First published on 10th November 2020
Abstract
Oxazolinyl- and arylchalcogenazolyl-substituted hydroxyfluorenes exhibiting excited-state intramolecular proton transfer (ESIPT) are described as potent and highly modular luminophores. Emission color tuning was achieved by varying the π-expansion and the insertion of different chalcogen atoms.
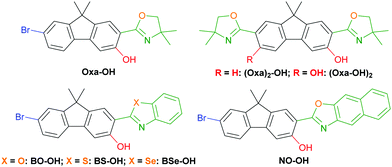
Excited-state intramolecular proton transfer (ESIPT)-based luminophores have attracted great attention due to their remarkable characteristics like large Stokes shifts (up to 12.000 cm−1) and dual emission1 behavior leading to white light generation.2,3 These properties derive from a four-level enol–keto-phototautomerism cycle, which comprise light absorption of the enol form, an ultrafast tautomerization,4 emission of the keto form and a cycle-completing second proton transfer. Extended application in electroluminescent devices,5 chemical sensing6 and biological imaging7 have been described. ESIPT-based fluorophores have a hydrogen bond donor in close proximity to a proton acceptor within an arene. While hydroxy groups are typically used as the hydrogen donors, the design of the proton acceptor is multifarious. Carbonyl groups,3,8 imines9 and benzazoles10 are well investigated proton acceptors, while the latter scaffold is widely used owing to its versatile synthesis and robustness towards harsh reaction conditions. Recently we demonstrated the efficiency of oxazolines as proton acceptors in minimalistic ESIPT-based fluorophores.11 While in these initial investigation simple mono- and benzo-fused cyclic arenes were used as the underlying π-scaffold, we were intended to expand oxazoline and oxazole-based ESIPT luminophores on fluorenes12 and other established fluorophores. Herein such oxazolinyl- and arylchalcogenazolyl-substituted hydroxyfluorenes as potent fluorophores (Fig. 1) are communicated. Seven fluorenes were synthesized and analyzed regarding their photophysical properties and crystal structures to gain a deep understanding towards their structure–property relationship.
Oxazoline-substituted hydroxyfluorenes Oxa-OH, (Oxa)2-OH and (Oxa-OH)2 were synthesized according to our previously reported procedures in very good yields.13,14 Hydroxylated benzoxazole (BO-OH), naphthoxazole (NO-OH), benzothiazole (BS-OH) and benzoselenazole (BSe-OH) were achieved using either a deprotonative metalation/aerobic hydroxylation method14 or a Pd-catalyzed hydroxylation with 1,4-dioxane as oxygen source.15
The optical properties of the respective precursor oxazolinyl- and arylchalcogenazolyl-substituted fluorenes were examined in different solvents (see the ESI,† chapter 3 and Table S2) as well as the final hydroxylated compounds which were additionally analyzed in their crystalline form. In the following, only the emission properties of hydroxylated fluorenes are discussed with a focus on the solid state (Table 1 and Table S3, ESI†).
| Compd | λ exc [nm] | λ em [nm] | Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) b [cm−1] b [cm−1] |
Φ F [%] | Φ F [%] | τ [ns] (Rel%)d | k r [108 s−1] | k nr [108 s−1] | x;y (CIE 1931) |
|---|---|---|---|---|---|---|---|---|---|
| a Additional spectroscopic data (samples and non-hydroxylated precursors in solution) is given in the ESI. b Excitation spectra of the crystalline samples were used for evaluation of the Stokes shift as absorption could not be measured due to low sample amount. c Quantum yields measured in CHCl3 solution. d Intensity-weighted relative ratios of the two decay components are given in parentheses if a bi-exponential fit was used. e k r and knr were calculated using the equations kr = ΦF/τ and knr = (1 − ΦF)/τ using the average lifetime. f Emission maximum of the enol form is given in parentheses. g The emission wavelength of the shoulder is given in parentheses. The amorphous sample is marked with an asterisk. | |||||||||
| Oxa-OH | 364 | 481 | 6680 | 38 | 17 | 2.9 | 1.32 | 2.13 | 0.160; 0.358 |
| (Oxa)2-OH | 370 | 485 | 6410 | 11 | 10 | 2.1 | 0.54 | 4.22 | 0.177; 0.420 |
| (Oxa-OH)2 | 380 | 493 | 6030 | 18 | 11 | 2.3 | 0.77 | 3.57 | 0.187; 0.469 |
| BO-OH | 397 | (452),f 520 | 5960 | 37 | 34 | 1.6 (28); 4.3 (72) | 1.04 | 1.78 | 0.259; 0.494 |
| NO-OH | 369 | 506, (546)g | 7340 | 3 | 38 | 1.6 (62); 3.8 (38) | 0.11 | 3.99 | 0.302; 0.554 |
| BS-OH | 450 | 575 | 4830 | 39 | 16 | 7.3 | 0.54 | 0.83 | 0.487; 0.501 |
| BSe-OH | 450 | 566 | 4550 | 7 | 6 | 1.7 | 0.41 | 5.47 | 0.459; 0.526 |
| BSe-OH* | 435 | 579 | 5720 | 7 | 1.8 | 0.39 | 5.04 | 0.510; 0.483 | |
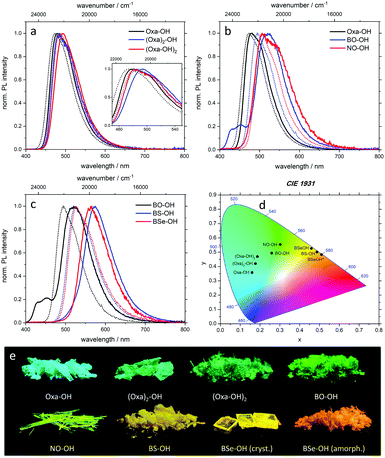
All target structures display emission in solution and solid state (Fig. 2a–e). The emission color of crystals formed from the oxazoline-substituted derivatives are in a close range from blue-cyan (481 nm, Oxa-OH) to green-cyan (485 nm, (Oxa)2-OH; 493 nm, (Oxa-OH2); Fig. 2a, e and Table 1). Switching the acceptor unit to π-extended benzo[d]oxazoles resulted in a bathochromic shift of λem to 520 nm for BO-OH and 506/546 nm for NO-OH (Fig. 2b, e and Table 1) and thus green to green-yellow emission. A more significant red-shift was observed when the oxygen of the benzo[d]oxazoles was substituted against heavier chalcogens. The corresponding benzothiazole (BS-OH) and benzoselenazole (BSe-OH) exhibit λem of 575 nm and 566 nm (Fig. 2c, e and Table 1). Interestingly, as the only substrate, amorphous BSe-OH exhibits a significant difference in emission color, since the keto emission band is broadened compared to crystalline BSe-OH resulting in a further bathochromic emission shift to 579 nm (Fig. 2d, e and Table 1). These observations indicate a continuous red-shift of emission maxima upon (1) π-expansion of the proton acceptor and (2) varying the heteroatom in the azole ring by walking down the chalcogenic group. This bathochromic shift can be explained by a decreased HOMO–LUMO gap.16
In order to compare the efficiency of the presented luminophores, the photoluminescence quantum yield ΦF was determined in the crystalline state. With ΦF = 38%, Oxa-OH has the highest emission efficiency of all oxazolinyl-substituted fluorenes. Interestingly, substitution of the bromine atom on the fluorene scaffold by a second oxazoline group ((Oxa2)-OH, 11%) leads to a decreased ΦF. Since heavy atoms like bromine facilitate intersystem crossing processes,17 the absence of such substituents should significantly increase ΦF. Incorporation of a second hydroxy group increased the emission efficiency slightly to 18% for (Oxa-OH)2. BO-OH and BS-OH exhibit almost similar quantum yields of 37% and 39%. A diminished ΦF was observed for π-expanded NO-OH (3%) and heavy atom incorporated BSe-OH (7%). Despite the possibility of phosphorescence generation through selenium incorporation, no long living species were detected in our studies. However, fluorescence lifetimes (τ) of the excited states were determined by time-correlated single photon counting (TCSPC), with values ranging from 1.7 ns for BSe-OH to 7.3 ns for BS-OH (Table 1 and Table S3, ESI†).
Due to the ESIPT character of the presented compounds, a large Stokes shift is expected. This is indeed the case with Stokes shifts ranging from 4550 cm−1 for BSe-OH to 7340 cm−1 for NO-OH in the solid state. Thus, efficient proton transfer in the excited state takes place resulting in exclusive keto emission. Also, the Stokes shift of the target structures in solution is in all cases significantly larger than the Stokes shift of the respective compounds prior to hydroxylation (see the ESI,† Tables S2 and S3). Apart from the large Stokes shift, ESIPT also manifests in dual emission behavior. For crystalline samples, only BO-OH exhibits a minor maximum at 452 nm, which is attributed to enol emission (Fig. 2b, c and Table 1). In case of diluted hydroxylated fluorenes, enol emission features are only detected for Oxa-OH, NO-OH and BS-OH in definite solvents (Fig. S3, S13 and S18, ESI†).
In addition, temperature-dependent emission measurements (from 77–200 K) were performed for BO-OH, BS-OH and BSe-OH (Fig. S15, S24 and S29, ESI†). In case of BO-OH an increase in luminescence intensity was observed upon cooling with a maximum intensity at 180 K (Fig. S15, ESI†). Such a behaviour is common for ESIPT luminophores, due to suppression of the proton transfer at deep temperatures.11,18BS-OH and BSe-OH, however, showed a steady increase of intensity during cooling with a maximum at 77 K (Fig. S24 and S29, ESI†). Moreover, at deep temperatures a more pronounced resolution of enol emission was achieved.
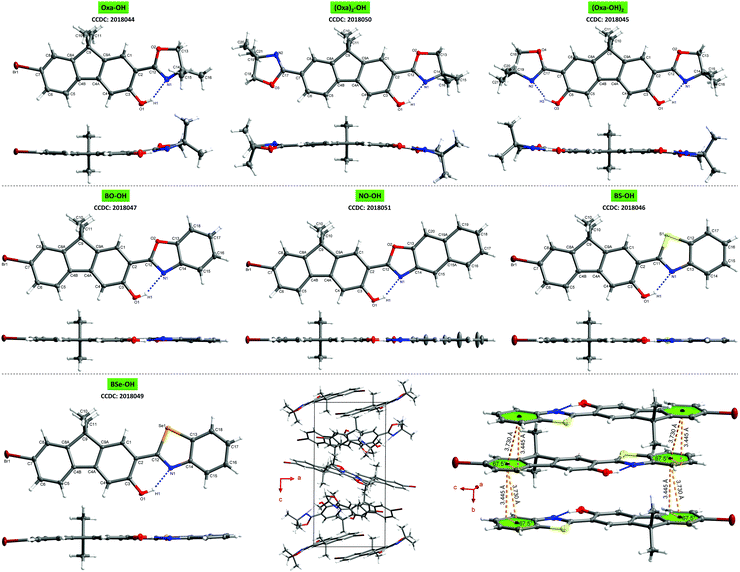
For gaining a better understanding of the structure–property relationship, single crystals of all seven ESIPT-luminophores were grown. A detailed analysis of the crystal properties is given in the ESI,† chapter 4.8. Despite the distorted structure of oxazoline-based fluorenes on the one hand and planar arylchalcogenazole-based fluorenes on the other hand (Fig. 3), no significant difference in emission efficiency is observed. Oxa-OH, BO-OH and BS-OH show similar quantum yields (Table 1), thus demonstrating the efficiency of oxazoline as potent proton acceptor in ESIPT-based fluorophores and an attractive alternative for widely used benzoxazoles and -thiazoles. The combination of a less-organized crystal lattice and good quantum yield in one system is extremely rare, since potent emitters often show extremely ordered crystal packing.11,19 Therefore, Oxa-OH represents an extraordinary case in which good quantum yields are achieved, although the molecular packing is highly unorganized.
In conclusion, ESIPT-based fluorenes varying in their proton acceptor groups have been synthesized and studied towards their photophysical properties to understand their structure–property relationship. Single crystal analysis revealed well-organized crystal lattices and planar molecular structures for arylchalcogenazolylfluorenes, whereas oxazolinyl-substituted fluorenes exhibit less-organized molecular packing and a distorted π-framework. Depending on the proton acceptor group, emission colors can be varied from blue-cyan to yellow in the crystal state. Quantum yields of up to almost 40% for Oxa-OH, BO-OH and BS-OH in the solid-state, demonstrates the efficiency of oxazoline as proton acceptor in ESIPT-based luminophores and as an alternative choice for arylchalcogenazoles. Furthermore, with Oxa-OH we present a potent emitter, whose molecular packing in the crystal lattice is highly unorganized compared to known strong luminophores.
N. C. B. and P. R. thank the DFG for partial funding under Germany's Excellence Strategy within the Cluster of Excellence PhoenixD (EXC 2122, Project ID 390833453) and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 714429).
Conflicts of interest
There are no conflicts to declare.References
- (a) D. Kim, K. Jeong, J. E. Kwon, H. Park, S. Lee, S. Kim and S. Y. Park, Nat. Commun., 2019, 10, 3089 CrossRef; (b) H.-Q. Yin, F. Yin and X.-B. Yin, Chem. Sci., 2019, 10, 11103–11109 RSC.
- (a) K. Benelhadj, W. Muzuzu, J. Massue, P. Retailleau, A. Charaf-Eddin, A. D. Laurent, D. Jacquemin, G. Ulrich and R. Ziessel, Chem. – Eur. J., 2014, 20, 12843–12857 CrossRef CAS; (b) H. Shono, T. Ohkawa, H. Tomoda, T. Mutai and K. Araki, ACS Appl. Mater. Interfaces, 2011, 3, 654–657 CrossRef CAS; (c) S. Park, J. E. Kwon, S. H. Kim, J. Seo, K. Chung, S.-Y. Park, D.-J. Jang, B. Milián Medina, J. Gierschner and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 14043–14049 CrossRef CAS.
- K.-C. Tang, M.-J. Chang, T.-Y. Lin, H.-A. Pan, T.-C. Fang, K.-Y. Chen, W.-Y. Hung, Y.-H. Hsu and P.-T. Chou, J. Am. Chem. Soc., 2011, 133, 17738–17745 CrossRef CAS.
- P. F. Barbara, P. K. Walsh and L. E. Brus, J. Phys. Chem., 1989, 93, 29–34 CrossRef CAS.
- (a) H. Yersin, Highly Efficient OLEDs with Phosphorescent Materials, Wiley-VCH, Hoboken, 2008 Search PubMed; (b) K. Müllen and U. Scherf, Organic Light-Emitting Devices. Synthesis, Properties, and Applications, Wiley-VCH, Weinheim, 2006 Search PubMed; (c) R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. D. C. Bradley, D. A. D. Santos, J. L. Brédas, M. Lögdlund and W. R. Salaneck, Nature, 1999, 397, 121–128 CrossRef CAS.
- (a) X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS; (b) L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993–1017 RSC; (c) S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339–1386 CrossRef CAS; (d) R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419–4476 CrossRef; (e) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS.
- (a) R. Long, C. Tang, J. Xu, T. Li, C. Tong, Y. Guo, S. Shi and D. Wang, Chem. Commun., 2019, 55, 10912–10915 RSC; (b) R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC; (c) H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS; (d) L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS.
- (a) A. Halder, B. Bhattacharya, F. Haque, S. Dinda and D. Ghoshal, Chem. – Eur. J., 2019, 25, 12196–12205 CrossRef CAS; (b) B. Liu, J. Wang, G. Zhang, R. Bai and Y. Pang, ACS Appl. Mater. Interfaces, 2014, 6, 4402–4407 CrossRef CAS; (c) A. S. Klymchenko and A. P. Demchenko, J. Am. Chem. Soc., 2002, 124, 12372–12379 CrossRef CAS.
- (a) Q. Jin, S. Chen, H. Jiang, Y. Wang, L. Zhang and M. Liu, Langmuir, 2018, 34, 14402–14409 CrossRef CAS; (b) P. Jagadesan, G. Eder and P. L. McGrier, J. Mater. Chem. C, 2017, 5, 5676–5679 RSC; (c) X. Ma, R. Sun, J. Cheng, J. Liu, F. Gou, H. Xiang and X. Zhou, J. Chem. Educ., 2016, 93, 345–350 CrossRef CAS; (d) A. Nano, M. P. Gullo, B. Ventura, N. Armaroli, A. Barbieri and R. Ziessel, Chem. Commun., 2015, 51, 3351–3354 RSC; (e) R. Wei, P. Song and A. Tong, J. Phys. Chem. C, 2013, 117, 3467–3474 CrossRef CAS.
- (a) M. Munch, M. Curtil, P. M. Vérité, D. Jacquemin, J. Massue and G. Ulrich, Eur. J. Org. Chem., 2019, 1134–1144 CrossRef CAS; (b) E. Heyer, K. Benelhadj, S. Budzák, D. Jacquemin, J. Massue and G. Ulrich, Chem. – Eur. J., 2017, 23, 7324–7336 CrossRef CAS; (c) S. Kothavale, Y. Erande and N. Sekar, ChemistrySelect, 2017, 2, 5013–5024 CrossRef CAS; (d) J. Ma, J. Zhao, P. Yang, D. Huang, C. Zhang and Q. Li, Chem. Commun., 2012, 48, 9720–9722 RSC.
- D. Göbel, D. Duvinage, T. Stauch and B. J. Nachtsheim, J. Mater. Chem. C, 2020, 8, 9213–9225 RSC.
- (a) V. S. Padalkar, Y. Tsutsui, T. Sakurai, D. Sakamaki, N. Tohnai, K. Kato, M. Takata, T. Akutagawa, K.-i. Sakai and S. Seki, J. Phys. Chem. B, 2017, 121, 10407–10416 CrossRef CAS; (b) V. S. Padalkar, D. Sakamaki, N. Tohnai, T. Akutagawa, K.-i. Sakai and S. Seki, RSC Adv., 2015, 5, 80283–80296 RSC; (c) A. R. Morales, K. J. Schafer-Hales, C. O. Yanez, M. V. Bondar, O. V. Przhonska, A. I. Marcus and K. D. Belfield, ChemPhysChem, 2009, 10, 2073–2081 CrossRef CAS; (d) S.-G. Roh, Y.-H. Kim, K. D. Seo, D. H. Lee, H. K. Kim, Y.-I. Park, J.-W. Park and J.-H. Lee, Adv. Funct. Mater., 2009, 19, 1663–1671 CrossRef CAS.
- (a) D. Göbel, M. Friedrich, E. Lork and B. J. Nachtsheim, Beilstein J. Org. Chem., 2020, 16, 1683–1692 CrossRef; (b) D. Göbel, N. Clamor and B. J. Nachtsheim, Org. Biomol. Chem., 2018, 16, 4071–4075 RSC.
- D. Göbel, N. Clamor, E. Lork and B. J. Nachtsheim, Org. Lett., 2019, 21, 5373–5377 CrossRef.
- K. Seth, M. Nautiyal, P. Purohit, N. Parikh and A. K. Chakraborti, Chem. Commun., 2015, 51, 191–194 RSC.
- A. Modelli, M. Guerra, D. Jones, G. Distefano, K. J. Irgolic, K. French and G. C. Pappalardo, Chem. Phys., 1984, 88, 455–461 CrossRef CAS.
- J. Xu, A. Takai, Y. Kobayashi and M. Takeuchi, Chem. Commun., 2013, 49, 8447–8449 RSC.
- Y. Zhang, H. Yang, H. Ma, G. Bian, Q. Zang, J. Sun, C. Zhang, Z. An and W.-Y. Wong, Angew. Chem., Int. Ed., 2019, 58, 8773–8778 CrossRef CAS.
- D. Yao, S. Zhao, J. Guo, Z. Zhang, H. Zhang, Y. Liu and Y. Wang, J. Mater. Chem., 2011, 21, 3568 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization data, photophysical data, X-ray crystallographic data, and copies of 1H-, 13C-NMR spectra. CCDC 2018044–2018047 and 2018049–2018051. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc05780k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |