Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spontaneous membrane-less multi-compartmentalization via aqueous two-phase separation in complex coacervate micro-droplets†
Nicolette G.
Moreau
 a,
Nicolas
Martin
a,
Nicolas
Martin
 b,
Pierangelo
Gobbo
b,
Pierangelo
Gobbo
 a,
T.-Y. Dora
Tang
a,
T.-Y. Dora
Tang
 c and
Stephen
Mann
c and
Stephen
Mann
 *ade
*ade
aCentre for Protolife Research and Centre for Organized Matter Chemistry, School of Chemistry, University of Bristol, Bristol BS8 1TS, UK. E-mail: s.mann@bristol.ac.uk
bUniv. Bordeaux, CNRS, Centre de Recherche Paul Pascal, UMR5031, 115 Avenue du Dr Albert Schweitzer, 33600 Pessac, France
cMax Planck Institute of Molecular Cell and Genetics, Pfotenhauerstrasse 108, 01307 Dresden, Germany
dBrisSynBio Synthetic Biology Research Centre, Life Sciences Building, University of Bristol, Tyndall Avenue, Bristol, BS8 1TQ, UK
eMax Planck Bristol Centre for Minimal Biology, School of Chemistry, University of Bristol, Bristol BS8 1TS, UK
First published on 10th September 2020
Abstract
Polyelectrolyte/nucleotide multiphase complex coacervate droplets are produced by internalized aqueous two-phase separation and used for the spatially dependent chemical transfer of sugar molecules, providing a step towards the development of membrane-free “organelles” within coacervate-based protocells.
Liquid–liquid phase separated micro-droplets produced by complex coacervation between oppositely charged polyions represent promising platforms to elucidate essential features of prebiotic chemical organization and emulate membrane-less organelles found in living cells.1,2 Coacervate droplets spontaneously sequester a wide range of functional solutes,1,3–5 and have been reported to assist protein folding,6 enhance enzyme activities7 and transcription/translation rates,8,9 and support RNA catalysis.10,11 Coacervate droplets can also dynamically respond to pH,1,12–15 temperature16 or light,17 but typically lack the morphological and functional heterogeneity of membrane-bounded protocell models. Significantly, membrane-less biological organelles have been reported to exhibit dynamic sub-structures including chemically-depleted vacuoles18 and immiscible chemically-rich liquid domains,19 which can facilitate sequential RNA processing by enzymes that localize in the distinct sub-compartments. Thus, achieving internal sub-structuration in synthetic coacervate droplets would provide a general strategy to spatially confine functional components into localized chemical reservoirs, dynamically segregate different biomolecules to distinct sub-compartments and orchestrate enzymatic reactions in space and time.
The formation of transient or stabilized water-filled vacuoles within complex coacervate droplets has been reported in association with osmotic pressure differences derived from in situ membrane formation,20–22 in droplets exposed to a continuous electric field23 and in the presence of a stoichiometric excess.24 The spontaneous formation of multiphase-separated droplets in simple coacervates of elastin-like polypeptides25 and in complex coacervates composed of multiple polyelectrolytes at equilibrium26–28 has been recently reported.
In this paper, we show that coacervate micro-droplets prepared by electrostatic complexation of poly(diallyldimethylammonium chloride) (PDDA; 8500 g mol−1, ∼53 monomer units) and adenosine triphosphate (ATP; 25 mM, pH 8) undergo vacuolization upon addition of a low molecular weight neutral molecule, tetraethyleneglycol (TEG; HO[CH2CH2O]3CH2CH2OH; 194.2 g mol−1). Vacuole formation is attributed to strong partitioning of TEG in the coacervate phase and subsequent partial dehydration of the PDDA/ATP matrix. We further demonstrate that addition of both TEG and high molecular weight dextran reconfigures the PDDA/ATP droplets into multiphase complex coacervate droplets by segregative aqueous phase separation. Finally, we test whether TEG/dextran phase separation within the PDDA/ATP droplets can be used to implement the spatially dependent chemical transfer of sugar molecules within the droplets. We sequester dextranase specifically within the coacervate shell and exploit the in situ partial hydrolysis of the polysaccharide to generate oligosaccharide fragments that spontaneously partition into the TEG-containing PDDA/ATP matrix. Taken together, our results demonstrate that the formation of both chemically depleted (water-filled) vacuoles and chemically enriched sub-compartments in complex coacervate micro-droplets can be implemented by controlling the uptake and aqueous two-phase separation of incompatible neutral molecules within the molecularly crowded medium.
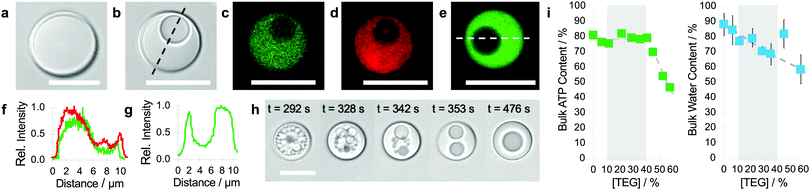
Complex coacervate microdroplets were prepared as a turbid suspension of an equimolar charge ratio mixture of PDDA and ATP in water (see Methods). The droplets were approximately 10 μm in diameter and appeared spherical and structurally homogeneous when viewed by bright field optical microscopy after settling on a glass slide (Fig. 1a). Injection of TEG (final concentration, typically 22 wt%) or a mixture of TEG and fluorescein isothiocyanate (FITC)-labelled TEG into the supernatant at a remote distance (0.5 cm) gave rise to the spontaneous formation of a single sub-compartment of different optical density within each droplet typically after 5 min (Fig. 1b). Confocal fluorescence microscopy images of the TEG-containing coacervate droplets doped with 2,4,6-trinitrophenol (TNP)-tagged ATP, rhodamine-isothiocyanate (RITC)-labelled PDDA or FITC-TEG revealed that the vacuoles were water-filled and essentially free of ATP, PDDA and TEG (Fig. 1c–g). Formation of the vacuoles occurred via the nucleation and coalescence of multiple sub-micrometre-sized compartments within the coacervate droplets (Fig. 1h and Fig. S1, Video S1, ESI†). The vacuoles moved freely within the viscoelastic PDDA/ATP matrix, slowly decreased in size over time and were expelled irreversibly into the supernatant solution after 10–20 min. Vacuolization was observed by microscopy across a wide range of TEG concentrations (22–36 wt%; Fig. S2, ESI†) with an upper limit set by the TEG-induced destabilization of the PDDA/ATP coacervate droplets. Minimal changes in ATP concentration occurred during vacuole formation although the water content decreased linearly as the TEG concentration increased (Fig. 1i). Taken together, the above results indicated that high amounts of TEG (ca. 90% of added molecules) could be accumulated within the PDDA/ATP droplets without inducing major changes in the composition of the coacervate phase. The high level of partitioning was indicative of preferential interactions between the host and guest components, which in turn resulted in microphase separation to produce water-filled vacuoles possibly via volume exclusion effects, decreases in water activity and surface tension changes coupled to dehydration of the polyionic complexes in the coacervate phase by osmotic pressure.29–31
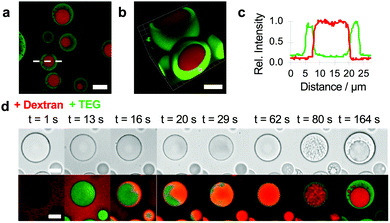
Given the ability of TEG to induce vacuolization within the PDDA/ATP coacervate droplets and undergo segregative liquid–liquid phase separation in the presence of high molecular weight dextran (Fig. S3, ESI†),32 we sought to exploit these processes to replace the molecularly depleted water-filled vacuoles with a chemically enriched sub-compartment. In so doing, we aimed to develop a spontaneous pathway to spatially organized droplets based on the confinement of aqueous two-phase separation within the matrix of an electrostatically matched complex coacervate. To achieve this goal, we mounted the PDDA/ATP coacervate droplets under water on a glass slide, added dextran (final concentration, 1.2 mg mL−1) with a molecular weight of 70 or 150 kDa, and then injected TEG (final concentration, 6 wt%) into the supernatant. Doping the coacervate droplets with TNP-ATP and RITC-PDDA and using unlabelled or fluorescently labelled TEG and dextran revealed the presence of a single well-defined sub-compartment in each droplet that was enriched in dextran and surrounded by a coacervate matrix containing ATP, PDDA and TEG (Fig. 2a–c). The free-moving dextran sub-compartments were stable within the coacervate droplets and were not readily expelled into the supernatant. Interestingly, real-time confocal fluorescence microscopy revealed that high molecular weight dextran was initially excluded from the coacervate droplets (Fig. S4, ESI†) and did not induce vacuolization. In contrast, when TEG was added rapidly (t < 13 s) to a dextran-containing suspension of PDDA/ATP droplets, the uniform partitioning of the oligoether into the coacervate phase triggered the uptake of dextran into the coacervate phase. Time-dependent fluorescence microscopy images revealed droplets that initially contained a homogeneous mixture of TEG and dextran, but which spontaneously demixed with time via aqueous two-phase separation to produce randomly distributed dextran-enriched nuclei that coalesced into a single dextran sub-compartment entrapped within the TEG-containing coacervate matrix (Fig. 2d and Fig. S5, Video S2, ESI†). Reducing the concentration of dextran inhibited coalescence of the incipient phase-separated nuclei to produce coacervate droplets with multiple quasi-stable sub-compartments (Fig. S6, ESI†). No dextran/TEG phase separation was observed within the droplets when a low molecular weight (4 kDa) dextran was used even though dextran was partitioned within the droplets in the absence of TEG (Fig. S7, ESI†).
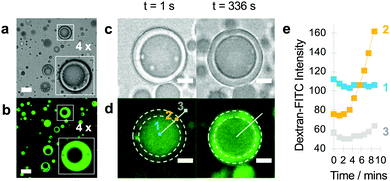
We tested whether TEG/dextran phase separation within the PDDA/ATP droplets could be used to implement the spatially dependent enzyme-mediated chemical transfer between the sub-compartment and surrounding coacervate matrix. As low molecular weight dextran was preferentially partitioned in the coacervate phase (Fig. S4, ESI†), we used in situ enzyme-mediated hydrolysis of the dextran sub-compartment to displace low molecular fragments of the polysaccharide reservoir into the enclosed TEG-containing coacervate matrix. To achieve this, we partitioned dextranase specifically into the TEG-enriched coacervate phase of droplets (Fig. 3a and b), and then monitored the localization and displacement of the polysaccharide fragments by using FITC-labelled dextran (70 kDa) as the guest molecule. Fluorescence microscopy images showed a progressive displacement of the green fluorescence intensity from the phase-separated sub-compartment to the coacervate phase typically within 5 min (Fig. 3c and d), which was consistent with line profiles recorded across individual droplets (Fig. 3e). We attributed these observations to the onset of dextranase-mediated partial hydrolysis of the polysaccharide chains into fluorescently tagged oligosaccharides, which in turn diffused across the phase boundary into the surrounding coacervate phase.
Taken together, our results demonstrate the ability of a small neutral molecule (TEG) to spontaneously induce the formation of multiphase complex coacervate droplets comprising water-filled vacuoles or dextran-enriched sub-compartments. Vacuolization occurs for added TEG concentrations between 22–36 wt% and is attributed to the coupling of changes in surface tension to partial dehydration of the PDDA/ATP coacervate phase by osmotic pressure. Interestingly, addition of either tetraethylene glycol monomethyl ether (TEG–CH3) or tetraethylene glycol dimethyl ether (CH3–TEG–CH3) did not induce vacuolization (Fig. S8, ESI†), suggesting that TEG-induced formation of the vacuoles was predominantly driven by hydrogen bonding and not hydrophobic interactions. In the presence of non-partitioned dextran, addition of low levels of TEG (6 wt%) to the continuous phase gives rise to co-sequestration followed by aqueous two-phase separation of the guest molecules to give a freely moving dextran sub-compartment embedded within a TEG-containing coacervate matrix. Interestingly, uptake of dextran is only initiated after the transfer of TEG from the continuous phase, suggesting that repulsive interactions between the PDDA/ATP matrix and polysaccharide macromolecules are attenuated in the presence of the oligoether. Moreover, as the concentrations of sequestered TEG and dextran are initially low, uptake of the guest molecules at first generates a homogeneous mixture within the droplets, which becomes unstable over time as the sequestered concentrations progressively increase. As the experimentally derived TEG partition coefficient at 6 wt% is approximately 14, we estimated a mean final concentration of TEG within the droplets of 3.6 M (coacervate volume fraction, 0.7%). Given this high level of sequestration, internalized demixing should occur once the dextran concentration attains a minimal value of 3 mg mL−1 (see Fig. S3, ESI†), equivalent to partitioning of approximately only 1.6% of the added dextran. Interestingly, no internalized segregative phase separation was observed when TEG was initially sequestered into the droplets followed by addition of high molecular weight dextran (Fig. S9, ESI†). Under these conditions, the dextran remained excluded from the droplet interior, presumably due to the high levels of pre-loaded TEG that inhibited mixing at the droplet/water interface.
Demixing of neutral molecules within the molecularly crowded complex coacervate at first sight seems surprising given the highly charged nature of the milieu but can be attributed to the reduced dielectric constant of the PDDA/ATP matrix,1 and the high level of incompatibility between the guest molecules.31 These factors also facilitate the sequestration of dextranase specifically into the TEG-containing molecular crowded matrix rather than the polysaccharide-enriched sub-compartment, and as a consequence enables the spatially dependent chemical transfer of sugar molecules within the multiphase droplets. This strategy offers a means to control the displacement of the internal reservoir across the internal phase boundary and could provide a route to the development of chemically dynamic “organelles” within coacervate-based protocell models.
In conclusion, our results demonstrate a simple and robust approach to spontaneous sub-compartmentalization within synthetic coacervate micro-droplets by rationally exploiting internalized processes of liquid–liquid phase separation. The work highlights new opportunities for the multi-phase restructuring of complex coacervate droplets and could provide a step towards the development of protocell models with subdivided interiors capable of programmable chemical trafficking.
This work was supported by the EPSRC (EP/G036780/1; Bristol Centre for Functional Nanomaterials), the ERC Advanced Grant Scheme (EC-2016-ADG 740235), BrisSynBio (BB/L01386X/1) and MaxSynBio Consortium (jointly funded by the Federal Ministry of Education and Research (Germany) and the Max Planck Society). We thank Dr S. Briggs for help with the preparation of RITC-PDDA.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Koga, D. S. Williams, A. W. Perriman and S. Mann, Nat. Chem., 2011, 3, 720–724 Search PubMed.
- N. Martin, ChemBioChem, 2019, 20, 2553–2568 Search PubMed.
- D. S. Williams, S. Koga, C. R. C. Hak, A. Majrekar, A. J. Patil, A. W. Perriman and S. Mann, Soft Matter, 2012, 8, 6004–6014 Search PubMed.
- T.-Y. D. Tang, M. Antognozzi, J. A. Vicary, A. W. Perriman and S. Mann, Soft Matter, 2013, 9, 7647–7656 Search PubMed.
- E. A. Frankel, P. C. Bevilacqua and C. D. Keating, Langmuir, 2016, 32, 2041–2049 Search PubMed.
- N. Martin, M. Li and S. Mann, Langmuir, 2016, 32, 5881–5889 Search PubMed.
- J. Crosby, T. Treadwell, M. Hammerton, K. Vasilakis, M. P. Crump, D. S. Williams and S. Mann, Chem. Commun., 2012, 48, 11832–11834 Search PubMed.
- E. Sokolova, E. Spruijt, M. M. K. Hansen, E. Dubuc, J. Groen, V. Chokkalingam, A. Piruska, H. A. Heus and W. T. S. Huck, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11692–11697 Search PubMed.
- T.-Y. D. Tang, D. van Swaay, A. deMello, J. L. R. Anderson and S. Mann, Chem. Commun., 2015, 51, 11429–11432 Search PubMed.
- B. Drobot, J. M. Iglesias-Artola, K. Le Vay, V. Mayr, M. Kar, M. Kreysing, H. Mutschler and T.-Y. D. Tang, Nat. Commun., 2018, 9, 3643 Search PubMed.
- R. R. Poudyal, R. M. Guth-Metzler, A. J. Veenis, E. A. Frankel, C. D. Keating and P. C. Bevilacqua, Nat. Commun., 2019, 10, 490 Search PubMed.
- D. Garenne, L. Beven, L. Navailles, F. Nallet, E. J. Dufourc and J.-P. Douliez, Angew. Chem., Int. Ed., 2016, 55, 13673–13677 Search PubMed.
- J. P. Douliez, N. Martin, C. Gaillard, T. Beneyton, J.-C. Baret, S. Mann and L. Beven, Angew. Chem., Int. Ed., 2017, 56, 13689–13693 Search PubMed.
- N. Martin, J.-P. Douliez, Y. Qiao, R. Booth, M. Li and S. Mann, Nat. Commun., 2018, 9, 3652 Search PubMed.
- C. Love, J. Steinkühler, D. T. Gonzales, N. Yandrapalli, T. Robinson, R. Dimova and T.-Y. D. Tang, Angew. Chem., Int. Ed., 2020, 59, 5950 Search PubMed.
- W. M. Aumiller Jr, F. P. Cakmak, B. W. Davis and C. D. Keating, Langmuir, 2016, 32, 10042–10053 Search PubMed.
- N. Martin, L. Tian, D. Spencer, A. Coutable-Pennarun, J. L. R. Anderson and S. Mann, Angew. Chem., Int. Ed., 2019, 58, 14594–14598 Search PubMed.
- H. B. Schmidt and R. Rohatgi, Cell Rep., 2016, 16, 1228–1236 Search PubMed.
- M. Feric, N. Vaidya, T. S. Harmon, D. M. Mitrea, L. Zhu, T. M. Richardson, R. W. Kriwacki, R. V. Pappu and C. P. Brangwynne, Cell, 2016, 165, 1686–1697 Search PubMed.
- D. W. Williams, A. J. Patil and S. Mann, Small, 2014, 10, 1830–1840 Search PubMed.
- L. Tian, M. Li, A. J. Patil, B. W. Drinkwater and S. Mann, Nat. Commun., 2019, 10, 3321 Search PubMed.
- P. Gobbo, L. Tian, P. B. V. V. S. Kumar, S. Turvey, M. Cattelan, A. J. Patil, M. Carraro, M. Bonchio and S. Mann, Nat. Commun., 2020, 11, 41 Search PubMed.
- Y. Yin, L. Niu, X. Zhu, M. Zhao, Z. Zhang, S. Mann and D. Liang, Nat. Commun., 2016, 7, 10658 Search PubMed.
- P. R. Banerjee, A. N. Milin, M. M. Moosa, P. L. Onuchic and A. A. Deniz, Angew. Chem., Int. Ed., 2017, 56, 11354–11359 Search PubMed.
- J. R. Simon, N. J. Carroll, M. Rubinstein, A. Chilkoti and G. P. Lopez, Nat. Chem., 2017, 9, 509–515 Search PubMed.
- G. A. Moutain and C. D. Keating, Biomacromolecules, 2020, 21, 630–640 Search PubMed.
- T. Lu and E. Spruijt, J. Am. Chem. Soc., 2020, 142, 2905–2914 Search PubMed.
- T. Kojima and S. Takayama, ACS Appl. Mater. Interfaces, 2018, 10, 32782–32791 Search PubMed.
- A. M. Marianelli, B. M. Miller and C. D. Keating, Soft Matter, 2018, 14, 368–378 Search PubMed.
- H. H. Hariri, A. M. Lehaf and J. B. Schlenoff, Macromolecules, 2020, 45, 9364–9372 Search PubMed.
- M. Gao, D. Gnutt, A. Orban, B. Appel, F. Righetti, R. Winter, F. Narberhaus, S. Muller and S. Ebbinghaus, Angew. Chem., Int. Ed., 2016, 55, 3224–3228 Search PubMed.
- C. D. Keating, Acc. Chem. Res., 2012, 45, 2114–2124 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experiments, optical and fluorescence microscopy images, phase diagram, partitioning data and supplementary movies. See DOI: 10.1039/d0cc05399f |
| This journal is © The Royal Society of Chemistry 2020 |