 Open Access Article
Open Access ArticleAn unusual Ni2Si2P8 cluster formed by complexation and thermolysis†
Christoph G. P.
Ziegler
 a,
Clemens
Taube
b,
John A.
Kelly
a,
Gabriele
Hierlmeier
a,
Maria
Uttendorfer
a,
Jan J.
Weigand
a,
Clemens
Taube
b,
John A.
Kelly
a,
Gabriele
Hierlmeier
a,
Maria
Uttendorfer
a,
Jan J.
Weigand
 *b and
Robert
Wolf
*b and
Robert
Wolf
 *a
*a
aUniversity of Regensburg, Institute of Inorganic Chemistry, 93040 Regensburg, Germany. E-mail: robert.wolf@ur.de
bTU Dresden, Faculty of Chemistry and Food Chemistry, 01062 Dresden, Germany. E-mail: jan.weigand@tu-dresden.de
First published on 27th October 2020
Abstract
[LSi(η2-P4)] (L = CH[C(Me)N(Dipp)][C(CH2)N(Dipp)], Dipp = 2,6-diisopropylphenyl) forms well-defined 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with N-heterocyclic carbene nickel fragments. The cluster compound [(IDipp)Ni2P8(SiL)2] (IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene) is selectively formed by thermolysis of the complex [(IDipp)Ni(μ-η2:2-P4)SiL].
1 complexes with N-heterocyclic carbene nickel fragments. The cluster compound [(IDipp)Ni2P8(SiL)2] (IDipp = 1,3-bis(2,6-diisopropylphenyl)imidazolin-2-ylidene) is selectively formed by thermolysis of the complex [(IDipp)Ni(μ-η2:2-P4)SiL].
Nickel phosphides are of interest in material science and industry due to their favourable magnetic and catalytic properties1 and their potential in hydrogen evolution reactions (HER),2 water splitting,3 hydrodenitrogenation and hydrodesulfuration reactions.4,5 Doping nickel phosphides with different transition metal elements is a common approach to modify their electro- and photocatalytic activity.6 Including main group elements, such as sulphur, has been shown to improve the catalytic activity of nickel phosphides in HER.7 Although promising, the synthesis of such metal phosphides remains challenging5,8 and contemporary methods require sensitive and toxic phosphine precursors (e.g. P(SiMe3)3), special additives (e.g. tri-n-octylphosphine, oleylamine) and high temperatures.9–11 A possible alternative is the mild thermolysis of carefully designed transition metal polyphosphido complexes,12 which can be accessed by the direct activation of white phosphorus (P4). The use of nickel(0) complexes already dates back to 1979, when Sacconi and co-workers reported the synthesis of a monohapto P4 complex [(κ3-P,P,P-NP3)Ni(η1-P4)] [(NP3 = tris(2-diphenylphosphinoethyl)amine)].13 Since then, several other groups have shown that Ni0 sources are suitable reagents for the targeted synthesis of nickel polyphosphido compounds.10,14–17 Le Floch and Mézailles prepared Ni2P nanoparticles directly by reacting [Ni(cod)2] (cod = 1,4-cyclooctadiene) with P4 in the presence of TOPO (tri-n-octylphosphine oxide) (Fig. 1, A)10 and Radius and co-workers accessed the P2 complex [{Ni(ImiPr2)2}2(μ-η2:2-P2)] (Fig. 1, B, ImiPr2 = 1,3-bis(iso-propyl)imidazolin-2-ylidene).16 More recently, our group described the aggregation of P4 using (NHC)Ni complexes leading to novel di- and trinuclear complexes (see e.g.Fig. 1, C).17
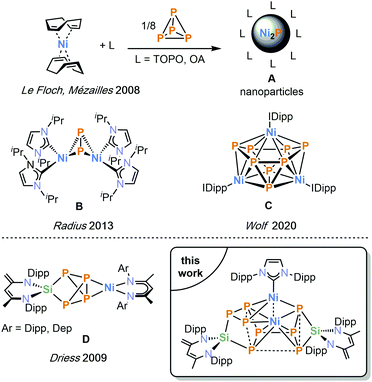 | ||
| Fig. 1 Examples of P4 activation by Ni0 precursors (top); heterodinuclear silicon–nickel polyphosphido complexes (bottom); Dipp = 2,6-iPr2C6H3, Dep = 2,6-Et2C6H3. | ||
Molecular main-group doped nickel phosphide complexes were reported by Driess in 2009 (Fig. 1, D).18 This type of complex is obtained from [LSi(η2-P4)]19 (L = CH[C(Me)N(Dipp)][C(CH2)N(Dipp)]) and [(L′Ni)2·toluene] (L′ = CH[CMeN(Dipp)]2) and [(L′′Ni)2·toluene] (L′′ = CH[CMeN(2,6-Et2C6H3)]2).18 The molecular structures of these complexes feature an intact SiP4 unit coordinating to the NiI centre.
We reasoned that reactions with a more strongly reducing Ni0 precursor could lead to P–P bond cleavage and subsequent aggregation of the resulting intermediates. Herein, we report the preparation of (NHC)Ni0 complexes with the tetraphosphasilatricyclopentane [LSi(η2-P4)]. Mild thermolysis of one of the complexes affords an unusual Ni2Si2P8 cluster by the nickel-mediated dimerization of two [LSi(η2-P4)] units.
Vinyltrimethylsilane complexes [(NHC)Ni(η2-vtms)2] (NHC = IDipp, IMes, vtms = Me3SiCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) were selected as well-proven “(NHC)Ni0” equivalents and reacted with [LSi(η2-P4)] in a 1
CH2) were selected as well-proven “(NHC)Ni0” equivalents and reacted with [LSi(η2-P4)] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry in toluene at −50 °C.19,20 Using two equivalents of [LSi(η2-P4)], [(NHC)Ni{(μ-η2:2-P4)SiL}2] 1a (NHC = IDipp) and 1b (NHC = IMes) are isolated as red powders in 50% and 26% yield, respectively (Scheme 1). A single crystal X-ray diffraction (XRD) study revealed that both compounds are isostructural and therefore only 1a will be discussed here. The molecular structure shows two [LSi(η2-P4)] ligands side-on coordinated to the bridging [(IDipp)Ni] unit. The P–P bond distances of the coordinated P atoms (P3–P4 2.2686(6), P7–P8 2.4749(7) Å) are considerably longer than the corresponding P–P bond length in [LSi(η2-P4)] (2.159(2) Å), although similar to complex D (2.351(3) Å).18,19 This might be explained by a significant back-donation of electron density from the Ni0 centre. The Ni–P distances (av. 2.2744 Å) in 1a lie in a similar range to those of D (av. 2.266 Å) (Fig. 2).18
2 stoichiometry in toluene at −50 °C.19,20 Using two equivalents of [LSi(η2-P4)], [(NHC)Ni{(μ-η2:2-P4)SiL}2] 1a (NHC = IDipp) and 1b (NHC = IMes) are isolated as red powders in 50% and 26% yield, respectively (Scheme 1). A single crystal X-ray diffraction (XRD) study revealed that both compounds are isostructural and therefore only 1a will be discussed here. The molecular structure shows two [LSi(η2-P4)] ligands side-on coordinated to the bridging [(IDipp)Ni] unit. The P–P bond distances of the coordinated P atoms (P3–P4 2.2686(6), P7–P8 2.4749(7) Å) are considerably longer than the corresponding P–P bond length in [LSi(η2-P4)] (2.159(2) Å), although similar to complex D (2.351(3) Å).18,19 This might be explained by a significant back-donation of electron density from the Ni0 centre. The Ni–P distances (av. 2.2744 Å) in 1a lie in a similar range to those of D (av. 2.266 Å) (Fig. 2).18
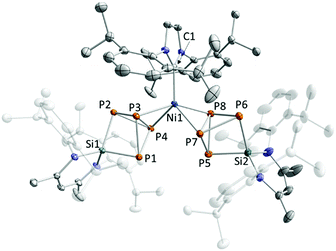 | ||
| Fig. 2 Solid-state molecular structure of 1a. Hydrogen atoms and solvate molecules are omitted for clarity; thermal ellipsoids are drawn at the 40% probability level; selected bond lengths [Å] for 1a: P1–P3 2.2651(6), P1–P4 2.2511(7), P2–P3 2.2565(7), P2–P4 2.2724(7), P3–P4 2.2686(6), Si1–P1 2.2512(8), Si1–P2 2.2422(7), Ni1–P3 2.2923(8), Ni1–P4 2.2674(8), P5–P7 2.2451(6), P5–P8 2.2603(7), P6–P7 2.2714(9), P6–P8 2.2607(7), P7–P8 2.4749(7), Si2–P5 2.2475(9), Si2–P6 2.2455(6), Ni1–P7 2.2657(6), Ni1–P8 2.2847(7), Ni1–C1 1.9761(16); bond distances and angles of derivatives 1b are presented in the ESI† (see Fig. S26). | ||
The 31P{1H} NMR spectra of 1a (Fig. S5, ESI†) shows two very broad resonances at δ = −234.3 ppm and 190.6 ppm with an integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which do not resolve at differing temperatures, suggesting a fluxional behaviour in solution (Fig. S7 and S8, ESI†). Complex 1b shows comparatively sharper multiplets at δ = −236.4 ppm and 194.2 ppm in the 31P{1H} NMR spectrum (Fig. S11, S13 and S14, ESI†). Despite the broad resonances observed in the 31P NMR spectra, both 1a and 1b give rise to well-resolved 1H NMR spectra (Fig. S3 and S9, ESI†), which indicate a symmetric structure. The 29Si NMR spectrum of 1a reveals a very broad signal at δ = −50.5 ppm (ν1/2 = 90 Hz), whereas 1b displays a broad pseudo-triplet resonance at δ = −51.1 ppm which is slightly shifted to higher field compared to the starting material [LSi(η2-P4)] (δ = −40.4 ppm).19
1, which do not resolve at differing temperatures, suggesting a fluxional behaviour in solution (Fig. S7 and S8, ESI†). Complex 1b shows comparatively sharper multiplets at δ = −236.4 ppm and 194.2 ppm in the 31P{1H} NMR spectrum (Fig. S11, S13 and S14, ESI†). Despite the broad resonances observed in the 31P NMR spectra, both 1a and 1b give rise to well-resolved 1H NMR spectra (Fig. S3 and S9, ESI†), which indicate a symmetric structure. The 29Si NMR spectrum of 1a reveals a very broad signal at δ = −50.5 ppm (ν1/2 = 90 Hz), whereas 1b displays a broad pseudo-triplet resonance at δ = −51.1 ppm which is slightly shifted to higher field compared to the starting material [LSi(η2-P4)] (δ = −40.4 ppm).19
The reaction with equimolar amounts of [(IDipp)Ni(η2-vtms)2] and [LSi(η2-P4)] in toluene at room temperature results in the selective formation of a new species [(IDipp)Ni(μ-η2:2-P4)SiL] (2). After filtration and recrystallisation from n-hexane, 2 was isolated as red crystalline blocks (Scheme 2). The solid-state structure of 2 reveals a heterodinuclear [Si(μ-η2:2-P4)Ni] core highly reminiscent of complex D (see the ESI† for full crystallographic data, NMR and bond analysis).18 When conducted with the less sterically demanding [(IMes)Ni(η2-vtms)2] the exclusive formation of 1b was observed.
Compound 2 slowly decomposes in solution and over the course of three weeks gives rise to a 31P{1H} NMR spectrum with two new ABCDEMSX spin systems, which are assigned to [(IDipp)Ni2P8(SiL)2] (3, vide infra). The clean formation of 3 can also be achieved by heating the reaction of [(IDipp)Ni(η2-vtms)2] and [LSi(η2-P4)] to 60 °C, circumventing the isolation of 2. Significant amounts of by-product IDipp were removed via sublimation (95 °C and 1 × 10−5 mbar). Subsequent recrystallization from toluene layered with n-hexane afforded compound 3 as brown crystals in a 33% yield.
The structure of 3 shows an unusual asymmetrical Ni2Si2P8 cluster with strongly varying P–P distances (range: 2.1702(8)–3.4219(8) Å). The phosphorus atoms P1, P3, P8, and P6 are aligned in a plane (torsion angle ∢ 1.8°) and coordinated by the central Ni1 atom. This plane is fused to a P3 ring (P2, P4 and P5) with one large P4⋯P5 distance of 2.5499(9) Å. In addition, this unit is connected to a second P3 ring (P6, P7 and P8) via a P–P single bond (P3–P7 2.1702(8) Å). The P8 framework is stabilised by two LSi moieties. Each silicon atom is connected to two phosphorus atoms. The whole framework is capped by a [(IDipp)Ni] fragment connected to three P atoms (P4, P5 and P7) (Fig. 3).
The Ni1–Ni2 distance (2.4126(4) Å) also deserves to be commented on. This value is consistent with an estimated value of 2.48 Å derived from the covalent radius of a single nickel atom (1.24 Å).21 While covalent metal–metal bonds are common for nickel(I) complexes,22 only a few related dinickel(0) complexes have been described with Ni0–Ni0 distances ranging from 2.437 to 2.572 Å.23 A notable example is the isonitrile complex [Ni2(μ-CNMe)(CNMe)2(μ-PPh2CH2PPh2)2] reported by Kubiak and co-workers with a Ni–Ni separation of 2.572(1) Å.23a
In order to investigate the bonding situation in more detail, the electronic structure was analysed by calculating intrinsic bond orbitals (IBOs)24 at the PBE/def2-TZVP level of theory. A truncated model of 3′ (with iPr of 3 replaced by Me groups) was used for the calculations. The composition of the IBOs suggests a 3d10 configuration for the two Ni atoms. Seven two-centre-two-electron P–P bonds, four doubly occupied IBOs involving the Si–P bonds and, additionally, three three-centre-two-electron bonds were calculated. One of the multicentre bonds (see Fig. S35, ESI†) suggests a Ni1⋯Ni2 interaction. The presence of a weak Ni–Ni bond is also supported by the calculated Mayer bond order of 0.3. The short Ni1⋯Ni2 distance might thus mainly be explained by the constrained alignment of the core of the cluster and additional significant contributions of the multicentre bonds.
Multinuclear NMR spectra of 3 suggest the presence of two isomers in solution in an approximate ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 due to the asymmetric L ligand. It is worth noting a similar ratio was found in the solid state, in the disorder of the diketiminate ligand (L) backbone (see Fig. S29, ESI†). As a result, the 1H, 13C NMR spectra are complex, but confirm the presence of all molecular components. The 29Si NMR spectrum shows two broad resonances at δ = −3.9 ppm and 60.8 ppm. However, the 31P{1H} NMR spectrum is particularly informative, showing two ABCDEMSX spin systems independent of temperature (see Fig. 4, Fig. S23 and S24, ESI†). To aid in assigning the signals, 31P and 29Si NMR chemical shieldings were calculated for the main isomer (see Tables S3 and S4, ESI†) of the slightly truncated model cluster 3′ at the TPSS/pcSseg-2 level of theory. Complex 2 was chosen as a reference system and the resonances of the 31P{1H} NMR spectrum (Fig. 4) were assigned according to the calculation, allowing for the iterative fitting of the 31P{1H} NMR spectrum of 3 (see the ESI†). DFT-calculated J(31P,31P) coupling constants were used as an initial starting point for the fitting procedure (see Table S1, ESI†). Five large 1J(31P,31P) coupling constants (−204.2 to −409.7 Hz) and one unexpectedly small 1J(31P,31P) coupling constant of −7.5 Hz between nuclei PA and PB were derived. The small 1J(PA,PB) (−7.5 Hz) coupling constant might be explained by the relatively long distance of the nuclei PA and PB observed by X-ray crystallography (P3–P8 2.4018(7) Å). Notably, a rather large coupling constant of 218.1 Hz between nuclei PD and PM is observed despite the long P–P distance (P1⋯P6 3.0176(7) Å) deduced from the solid-state structure. We reason this finding as a through space coupling as observed also in other polyphosphorus compounds.25
1 due to the asymmetric L ligand. It is worth noting a similar ratio was found in the solid state, in the disorder of the diketiminate ligand (L) backbone (see Fig. S29, ESI†). As a result, the 1H, 13C NMR spectra are complex, but confirm the presence of all molecular components. The 29Si NMR spectrum shows two broad resonances at δ = −3.9 ppm and 60.8 ppm. However, the 31P{1H} NMR spectrum is particularly informative, showing two ABCDEMSX spin systems independent of temperature (see Fig. 4, Fig. S23 and S24, ESI†). To aid in assigning the signals, 31P and 29Si NMR chemical shieldings were calculated for the main isomer (see Tables S3 and S4, ESI†) of the slightly truncated model cluster 3′ at the TPSS/pcSseg-2 level of theory. Complex 2 was chosen as a reference system and the resonances of the 31P{1H} NMR spectrum (Fig. 4) were assigned according to the calculation, allowing for the iterative fitting of the 31P{1H} NMR spectrum of 3 (see the ESI†). DFT-calculated J(31P,31P) coupling constants were used as an initial starting point for the fitting procedure (see Table S1, ESI†). Five large 1J(31P,31P) coupling constants (−204.2 to −409.7 Hz) and one unexpectedly small 1J(31P,31P) coupling constant of −7.5 Hz between nuclei PA and PB were derived. The small 1J(PA,PB) (−7.5 Hz) coupling constant might be explained by the relatively long distance of the nuclei PA and PB observed by X-ray crystallography (P3–P8 2.4018(7) Å). Notably, a rather large coupling constant of 218.1 Hz between nuclei PD and PM is observed despite the long P–P distance (P1⋯P6 3.0176(7) Å) deduced from the solid-state structure. We reason this finding as a through space coupling as observed also in other polyphosphorus compounds.25
 | ||
| Fig. 4 31P{1H} NMR spectrum of cluster 3 in C6D6 at room temperature with nuclei assigned to the ABCDEMSX spin system; two isomers are present in solution (signals colour-coded in black and red, assigned based on integration); chemical shifts for isomer A: δ/ppm = −237.1 (PA), −190.3 (PB), −162.2 (PC), −149.2 (PD), −123.5 (PE), −60.5 (PM), 51.3 (PS), 191.1(PX); isomer B: δ/ppm −232.2 (PA), −199.3 (PB), −164.8 (PC), −145.0 (PD), −113.7 (PE), −46.5 (PM), 46.4 (PS), 208.8 (PX); DFT-calculated and simulated coupling constants are presented in the ESI† (see Table S1); insets: representation of the core of the cluster; thermal ellipsoids are drawn at the 40% probability level. | ||
In summary, we have shown that the (NHC)Ni synthon [(NHC)Ni(η2-vtms)2] (NHC = IDipp, IMes, vtms = Me3SiCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) effects a unusual dimerisation of [LSi(η2-P4)] to form the Ni2Si2P8 cluster 3. Additionally, the classical (NHC)Ni complexes 1a, b and 2 have been isolated alongside. Such Ni complexes show great potential as starting materials for the synthesis of ternary phosphorus cluster such as compound 3 as they are well-defined and conveniently prepared. Derivatisation reactions of the cluster core 3 through the substitution of the diketiminate ligands L may further enhance the diversity of this class of cluster molecules. An extension of the synthetic methodology reported here and the use of 3 and related clusters as single source precursors for phosphorus-based materials will be of significant future interest.
CH2) effects a unusual dimerisation of [LSi(η2-P4)] to form the Ni2Si2P8 cluster 3. Additionally, the classical (NHC)Ni complexes 1a, b and 2 have been isolated alongside. Such Ni complexes show great potential as starting materials for the synthesis of ternary phosphorus cluster such as compound 3 as they are well-defined and conveniently prepared. Derivatisation reactions of the cluster core 3 through the substitution of the diketiminate ligands L may further enhance the diversity of this class of cluster molecules. An extension of the synthetic methodology reported here and the use of 3 and related clusters as single source precursors for phosphorus-based materials will be of significant future interest.
We thank Dr Peter Coburger for assistance with the DFT calculations and Julia Leitl for help with preparing the manuscript. Generous financial support by the Deutsche Forschungsgemeinschaft (WE4621/3-1 and WO1496/7-1), the European Research Council (CoG 772299) and the Fonds der Chemischen Industrie (Kekulé fellowship to G.H.) is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- V. Jourdain, E. T. Simpson, M. Paillet, T. Kasama, R. E. Dunin-Borkowski, P. Poncharal, A. Zahab, A. Loiseau, J. Robertson and P. Bernier, J. Phys. Chem. B, 2006, 110, 9759 CrossRef CAS.
- (a) H. Li, G. Li and Z. Liu, ACS Omega, 2019, 4, 2075 CrossRef CAS; (b) A. Parra-Puerto, K. L. Ng, K. Fahy, A. E. Goode, M. P. Ryan and A. Kucernak, ACS Catal., 2019, 9, 11515 CrossRef CAS; (c) Z. Sun, M. Zhu, M. Fujitsuka, A. Wang, C. Shi and T. Majima, ACS Appl. Mater. Interfaces, 2017, 9, 30583 CrossRef CAS; (d) Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529 RSC.
- G. Zhang, G. Wang, Y. Liu, H. Liu, J. Qu and J. Li, J. Am. Chem. Soc., 2016, 138, 14686 CrossRef CAS.
- (a) S. T. Oyama, T. Gott, H. Zhao and Y.-K. Lee, Catal. Today, 2009, 143, 94 CrossRef CAS; (b) I. I. Abu and K. J. Smith, Appl. Catal., A, 2007, 328, 58 CrossRef CAS; (c) S. Yang, C. Liang and R. Prins, J. Catal., 2006, 237, 118 CrossRef CAS; (d) Y. Shu and S. T. Oyama, Chem. Commun., 2005, 1143 RSC; (e) S. T. Oyama, J. Catal., 2003, 216, 343 CrossRef CAS; (f) R. Prins, G. Pirngruber and T. Weber, Chimia, 2001, 55, 791 CAS.
- S. L. Brock, S. C. Perera and K. L. Stamm, Chem. – Eur. J., 2004, 10, 3364 CrossRef CAS.
- (a) Z. Jin, P. Li, X. Huang, G. Zeng, Y. Jin, B. Zheng and D. Xiao, J. Mater. Chem. A, 2014, 2, 18593–18599 RSC; (b) Y. Li, X. Jiang, Z. Miao, J. Tang, Q. Zheng, F. Xie and D. Lin, ChemCatChem, 2019, 12, 917–925 CrossRef; (c) H.-W. Man, C.-S. Tsang, M. Meng-Jung Li, J. Mo, B. Huang, L. Yoon Suk Lee, Y.-C. Leung, K.-Y. Wong and S. Chi Edman Tsang, Appl. Catal., B, 2019, 242, 186–193 CrossRef CAS; (d) S. Anantharaj, S. Rao Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6(12), 8069–8097 CrossRef CAS.
- J. Chang, K. Li, Z. Wu, J. Ge, C. Liu and W. Xing, ACS Appl. Mater. Interfaces, 2018, 10, 26303–26311 CrossRef CAS.
- M. Shatruk, in Fundamentals and applications of phosphorus nanomaterials, ed. H.-F. Ji, American Chemical Society, Washington, DC, 2019, vol. 6, pp. 103–134 Search PubMed.
- (a) K. L. Stamm, J. C. Garno, G.-y. Liu and S. L. Brock, J. Am. Chem. Soc., 2003, 125, 4038 CrossRef CAS; (b) S. C. Perera, G. Tsoi, L. E. Wenger and S. L. Brock, J. Am. Chem. Soc., 2003, 125, 13960 CrossRef CAS.
- S. Carenco, I. Resa, X. Le Goff, P. Le Floch and N. Mézailles, Chem. Commun., 2008, 2568 RSC.
- (a) A. E. Henkes, Y. Vasquez and R. E. Schaak, J. Am. Chem. Soc., 2007, 129, 1896 CrossRef CAS; (b) R.-K. Chiang and R.-T. Chiang, Inorg. Chem., 2007, 46, 369 CrossRef CAS.
- (a) T. Grell, D. M. Yufanyi, A. K. Adhikari, M.-B. Sárosi, P. Lönnecke and E. Hey-Hawkins, Pure Appl. Chem., 2019, 91, 103 CAS; (b) A. Kırcalı Akdag, P. Lönnecke and E. Hey-Hawkins, Z. Anorg. Allg. Chem., 2014, 640, 271 CrossRef.
- P. Dapporto, S. Midollini and L. Sacconi, Angew. Chem., Int. Ed. Engl., 1979, 18, 469 CrossRef.
- Selected examples of P4 activation using NiII sources: (a) M. Di Vaira, S. Midollini and L. Sacconi, J. Am. Chem. Soc., 1979, 101, 1757 CrossRef CAS; (b) M. Di Vaira, C. A. Ghilardi, S. Midollini and L. Sacconi, J. Am. Chem. Soc., 1978, 100, 2550 CrossRef CAS.
- Selected examples of P4 activation using NiI sources: (a) S. Pelties, D. Herrmann, B. d. Bruin, F. Hartl and R. Wolf, Chem. Commun., 2014, 50, 7014 RSC; (b) S. Yao, Y. Xiong, C. Milsmann, E. Bill, S. Pfirrmann, C. Limberg and M. Driess, Chem. – Eur. J., 2010, 16, 436 CrossRef CAS; (c) O. J. Scherer, J. Braun, P. Walther and G. Wolmershäuser, Chem. Ber., 1992, 125, 2661 CrossRef CAS; (d) O. J. Scherer, J. Braun and G. Wolmershäuser, Chem. Ber., 1990, 123, 471 CrossRef CAS; (e) O. J. Scherer, T. Dave, J. Braun and G. Wolmershäuser, J. Organomet. Chem., 1988, 350, C20–C24 CrossRef CAS.
- B. Zarzycki, T. Zell, D. Schmidt and U. Radius, Eur. J. Inorg. Chem., 2013, 2051 CrossRef CAS.
- G. Hierlmeier, P. Coburger, N. P. van Leest, B. de Bruin and R. Wolf, Angew. Chem., Int. Ed., 2020, 59, 14148–14153 CrossRef CAS.
- Y. Xiong, S. Yao, E. Bill and M. Driess, Inorg. Chem., 2009, 48, 7522 CrossRef CAS.
- Y. Xiong, S. Yao, M. Brym and M. Driess, Angew. Chem., Int. Ed., 2007, 46, 4511 CrossRef CAS.
- (a) M. R. Elsby, J. Liu, S. Zhu, L. Hu, G. Huang and S. A. Johnson, Organometallics, 2019, 38, 436 CrossRef CAS; (b) M. R. Elsby and S. A. Johnson, J. Am. Chem. Soc., 2017, 139, 9401 CrossRef CAS.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832 RSC.
- J.-C. Hierso, Chem. Rev., 2014, 114, 4838 CrossRef CAS.
- (a) D. L. DeLaet, P. E. Fanwick and C. P. Kubiak, Organometallics, 1986, 5, 1807 CrossRef CAS; (b) A. Kempter, C. Gemel, T. Cadenbach and R. A. Fischer, Organometallics, 2007, 26, 4257 CrossRef CAS; (c) O. Serrano, E. Hoppe, J. C. Fettinger and P. P. Power, J. Organomet. Chem., 2011, 696, 2217 CrossRef CAS; (d) A. Seifert and G. Linti, Inorg. Chem., 2008, 47, 11398 CrossRef CAS.
- G. Knizia, J. Chem. Theory Comput., 2013, 9, 4834 CrossRef CAS.
- For representative examples, see: (a) C. Taube, K. Schwedtmann, M. Noikham, E. Somsook, F. Hennersdorf, R. Wolf and J. J. Weigand, Angew. Chem., Int. Ed., 2020, 59, 3585 CrossRef CAS; (b) P. Coburger, P. Bielytskyi, D. Williamson, E. Rys, A. Kreienbrink, P. Lönnecke, J. Matysik and E. Hey-Hawkins, Chem. – Eur. J., 2019, 25, 11456 CrossRef CAS; (c) H. C. E. McFarlane and W. McFarlane, Polyhedron, 1999, 18, 2117 CrossRef CAS; (d) H. C. E. McFarlane and W. McFarlane, Polyhedron, 1988, 7, 1875 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2009284–2009287. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc05365a |
| This journal is © The Royal Society of Chemistry 2020 |



