Cut and sew: benzofuran-ring-opening enabled cyclopentenone ring formation†
Abstract
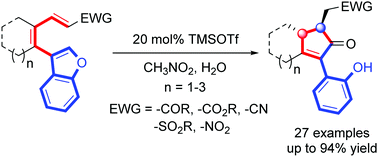
A facile approach to the fully substituted cyclopentenones involving an unprecedented benzofuran-ring-opening is described. The cleavage of a benzofuran endocyclic C2–O bond proceeded smoothly in the absence of any transition metal catalyst or highly reactive organometallic reagent. Such benzofuran-ring-opening is delicately incorporated into an acid-catalyzed cascade process, orchestrating a novel synthetic strategy for complex cyclopentenones with excellent yields and diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...