Facile one-pot synthesis of cyclic peptide-conjugated photosensitisers for targeted photodynamic therapy†
Abstract
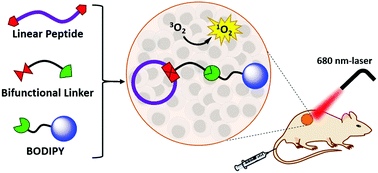
A novel synthetic strategy for in situ cyclisation of peptides and conjugation with functional boron dipyrromethenes (BODIPYs) has been developed. Linear peptides with up to 16 amino acid residues can be cyclised effectively and the resulting conjugates can be isolated in higher than 20% yield. One of the conjugates having a cyclic RGD moiety has been studied both in vitro and in vivo. It exhibits high and selective affinity towards the αvβ3-positive cell lines and induces high photocytotoxicity. The conjugate can also selectively localise in and effectively inhibit the growth of αvβ3-overexpressed tumour in vivo.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...