Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A large kinetic isotope effect in the reaction of ascorbic acid with 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO˙) in aqueous buffer solutions†
Ikuo
Nakanishi
 *a,
Yoshimi
Shoji
a,
Kei
Ohkubo
*a,
Yoshimi
Shoji
a,
Kei
Ohkubo
 ab,
Toshihiko
Ozawa
c,
Ken-ichiro
Matsumoto
ab,
Toshihiko
Ozawa
c,
Ken-ichiro
Matsumoto
 a and
Shunichi
Fukuzumi
a and
Shunichi
Fukuzumi
 *de
*de
aQuantitative RedOx Sensing Group, Department of Basic Medical Sciences for Radiation Damages, National Institute of Radiological Sciences (NIRS), Quantum Medical Science Directorate, National Institutes for Quantum and Radiological Science and Technology (QST), Inage-ku, Chiba 263-8555, Japan. E-mail: nakanishi.ikuo@qst.go.jp; Fax: +81-43-255-6819; Tel: +81-43-206-3131
bInstitute for Advanced Co-Creation Studies, Open and Transdisciplinary Research Initiatives, Osaka University, 2-8 Yamada-oka, Suita, Osaka 565-0871, Japan
cNihon Pharmaceutical University, Kitaadachi-gun, Saitama 362-0806, Japan
dDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 03760, Korea
eFaculty of Science and Engineering, Meijo University, Nagoya, Aichi 468-8502, Japan
First published on 24th August 2020
Abstract
A large kinetic isotope effect (KIE, kH/kD) of 12.8 was observed for the hydrogen-transfer reaction from ascorbic acid to 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO˙) in a phosphate buffer solution (0.05 M, pH/pD 7.0) at 298 K. The isotopic difference in the activation energies (6.8 kJ mol−1) determined from the temperature dependence of the KIE suggests that quantum mechanical tunneling may partly play a role in the reaction, although the isotopic ratio of the Arrhenius prefactor (AH/AD = 0.86) is within the semiclassical limits.
Quantum mechanical tunneling in hydrogen-transfer reactions1–3 in biological redox systems has attracted considerable attention with regard to the quantum mechanical behaviour in biology in recent years.4 Uršić et al. reported that a large kinetic isotope effect (KIE, kH/kD) of 24.2 was observed in water for the hydrogen-transfer reaction from ascorbic acid (AscH2), one of the representative water-soluble antioxidants, to 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) radicals.5 This is the first report about hydrogen tunneling in a reaction involving AscH2. On the other hand, Li has recently reported a new and simple antioxidant assay in vitro using 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radicals (PTIO˙), one of the nitronyl nitroxide radicals, where a hydrogen transfer occurred from antioxidants to PTIO˙.6 However, little is known about the kinetics of the reaction between antioxidants and PTIO˙, as well as the KIE. We report herein the observation of a large kinetic isotope effect for the reaction of AscH2 with PTIO˙ in a phosphate buffer. A possibility of the involvement of quantum mechanical tunneling is also discussed based on the temperature dependence of the KIE.
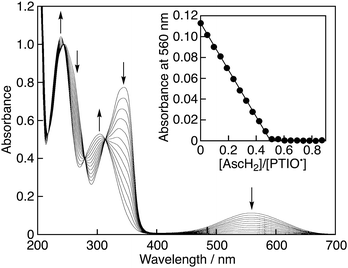
When AscH2 was added to the phosphate buffer solution (0.05 M, pH 7.0) of PTIO˙, the bands at 345 and 560 nm decreased immediately with clear isosbestic points at 218, 244, 279 and 313 nm as shown in Fig. 1. Since the pKa value of AscH2 is reported to be 4.1,7 AscH2 undergoes deprotonation and exists in its anionic form, AscH−, in phosphate buffer solution (0.05 M, pH 7.0). Thus, this spectral change indicates that AscH− efficiently scavenged PTIO˙ in phosphate buffer. The spectral titration (inset of Fig. 1) shows that the stoichiometry of the reaction is given by eqn (1), where AscH− reacts with 2PTIO˙.
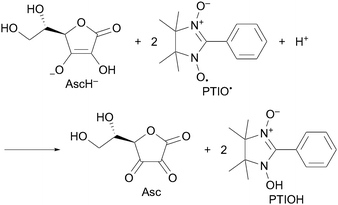 | (1) |
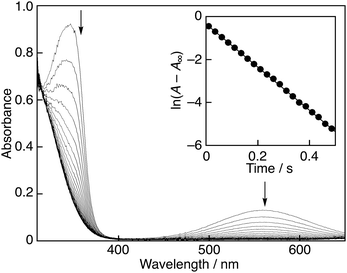
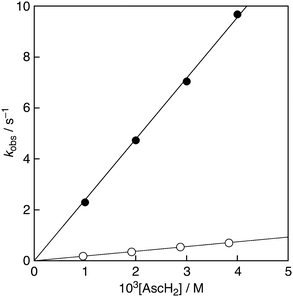
The decay of the absorbance at 560 nm monitored by a stopped-flow technique obeyed pseudo-first-order kinetics, when the concentration of AscH2 ([AscH2]) was maintained at more than a 10-fold excess of PTIO˙ concentration (Fig. 2). The pseudo-first-order rate constants (kobs) linearly increased with increasing [AscH2] (Fig. 3). From the slope of the linear plot, the second-order rate constant (kH) for the scavenging reaction of PTIO˙ by AscH2 [eqn (2)] was determined in a phosphate buffer (0.05 M, pH 7.0) to be 2.4 × 103 M−1 s−1. This value is smaller than that determined for the reaction between AscH2 and β-cyclodextrin-solubilised 2,2-diphenyl-1-picrylhydrazyl radicals (DPPH˙)8 under the same experimental conditions (kH = 5.6 × 103 M−1 s−1). Thus, the reactivity of PTIO˙ toward AscH2 is lower than that of DPPH˙.
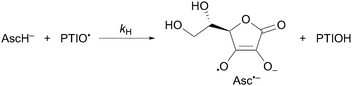 | (2) |
When H2O was replaced by D2O to prepare the phosphate buffer, the exchangeable O–H protons in AscH2 are replaced by deuterons from D2O to produce AscD2. The second-order rate constant (kD) thus determined for the reaction of AscD2 with PTIO˙ was significantly decreased to be 1.9 × 102 M−1 s−1. Thus, the KIE (kH/kD) is calculated to be 12.8. Such a large KIE value has clearly precluded an electron-transfer pathway in the oxidation reaction of AscH2 by PTIO˙. This value is beyond the maximum expected semiclassical value of 7.9 for the dissociation of the O–H bond.9 We also performed the reaction of AscH2 with PTIO˙ in the temperature range from 288 to 308 K and the kH and kD values were determined from the slopes of the linear plots of the kobs values vs. concentrations of AscH2 or AscD2 (Table 1).
| T/K | k H/M−1 s−1 | k D/M−1 s−1 | k H/kD |
|---|---|---|---|
| 288 | 1.5 × 103 | 9.6 × 10 | 15.6 |
| 293 | 2.0 × 103 | 1.5 × 102 | 13.3 |
| 298 | 2.4 × 103 | 1.9 × 102 | 12.8 |
| 300 | 2.8 × 103 | 1.9 × 102 | 14.8 |
| 303 | 2.9 × 103 | 2.2 × 102 | 13.3 |
| 308 | 3.1 × 103 | 2.6 × 102 | 12.2 |
Furthermore, as the Arrhenius plots are shown in Fig. 4, linear correlations of ln kHvs. T−1 and ln kDvs. T−1 were observed in the reaction of AscH2 with PTIO˙ in the whole temperature range. From the intercepts of Fig. 4, the isotopic ratio of the Arrhenius prefactor (AH/AD = 0.86) was obtained. This value can be fitted within the semiclassical limits of 0.7–1.4 for the AH/AD value in a hydrogen-transfer process.9 The isotopic difference in the activation energies Ea(D)–Ea(H) was 6.8 kJ mol−1, which is beyond the difference in zero-point energies of 5.1 kJ mol−1.9 A large AH/AD value (≫1) in hydrogen transfer of some enzymes has been reported by Klinman et al., proposing a full tunneling mode to explain such observation.3 Furthermore, Uršić et al. claimed that quantum mechanical tunneling plays a role in the reaction between AscH2 and TEMPO in water–dioxane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) based on the large KIE value of 31.1 and Ea(D)–Ea(H) value of 8.2 kJ mol−1, although the AH/AD value is 1.2.5 Thus, quantum mechanical tunneling may partly play a role in the reaction between AscH2 and PTIO˙ in a phosphate buffer solution.
1 v/v) based on the large KIE value of 31.1 and Ea(D)–Ea(H) value of 8.2 kJ mol−1, although the AH/AD value is 1.2.5 Thus, quantum mechanical tunneling may partly play a role in the reaction between AscH2 and PTIO˙ in a phosphate buffer solution.
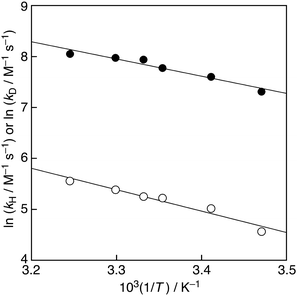 | ||
| Fig. 4 Arrhenius plots of ln kHvs. T−1 (closed circles) and ln kDvs. T−1 (open circles) in phosphate buffer (H2O, 0.05 M, pH 7.0) and in phosphate buffer (D2O, 0.05 M, pD 7.0), respectively. | ||
In summary, a large KIE was observed for the hydrogen-transfer reaction from AscH2 to PTIO˙. The temperature dependence of the KIE suggests that quantum mechanical tunneling may partly play a role in the reaction, although the isotopic ratio of the Arrhenius prefactor (AH/AD) is within the semiclassical limits. Because there are only a few reports about a large KIE in a reaction involving AscH2, this study provides valuable information for the biological redox reactions including ascorbic acid.
This work was partially supported by Grant-in-Aid (No. 18K06620 to I. N., 20H02779, 20H04819, 18H04650, 17H03010 and 16H02268 to K. O.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We also thank Dr Hiroaki Kotani (University of Tsukuba) for valuable discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Meisner and J. Kästner, Angew. Chem., Int. Ed., 2016, 55, 5400 CrossRef CAS.
- P. R. Schreiner, J. Am. Chem. Soc., 2017, 139, 15276 CrossRef CAS.
- (a) J. P. Klinman and A. R. Offenbacher, Acc. Chem. Res., 2018, 51, 1966 CrossRef CAS; (b) Z. D. Nagel and J. P. Klinman, Nat. Chem. Biol., 2009, 5, 543 CrossRef CAS.
- J. McFadden and J. Al-Khalili, Proc. R. Soc. A, 2018, 474, 20180674 CrossRef.
- (a) I. Sajenko, V. Pilepić, C. Jakobušić Brala and S. Uršić, J. Phys. Chem. A, 2010, 114, 3423 CrossRef CAS; (b) A. Karković Marković, C. Jakobušić Brala, V. Pilepić and S. Uršić, Molecules, 2020, 25, 1443 CrossRef.
- X. Li, J. Agric. Food Chem., 2017, 65, 6288 CrossRef CAS.
- (a) C. Creutz, Inorg. Chem., 1981, 20, 4449 CrossRef CAS; (b) N. H. Williams and J. K. Yandell, Aust. J. Chem., 1982, 35, 1133 CrossRef CAS.
- (a) I. Nakanishi, K. Ohkubo, K. Imai, M. Kamibayashi, Y. Yoshihashi, K. Matsumoto, K. Fukuhara, K. Terada, S. Itoh, T. Ozawa and S. Fukuzumi, Chem. Commun., 2015, 51, 8311 RSC; (b) I. Nakanishi, K. Ohkubo, M. Kamibayashi, Y. Ogawa, T. Ozawa, K. Matsumoto and S. Fukuzumi, ChemistrySelect, 2016, 1, 3367 CrossRef CAS.
- R. P. Bell, The Tunnel Effect in Chemistry, Chapman & Hall, London, 1980 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/d0cc05214k |
| This journal is © The Royal Society of Chemistry 2020 |