Open Access Article
Open Access ArticleCu(II)-Catalysed β-silylation of dehydroalanine residues in peptides and proteins†
Reinder H.
de Vries
and
Gerard
Roelfes
 *
*
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: J.G.Roelfes@rug.nl
First published on 10th August 2020
Abstract
We report the efficient and selective Cu(II)-catalysed β-silylation of naturally occurring dehydroalanine (Dha) residues in various ribosomally synthesized and post-translationally modified peptides (RiPPs). The method is also applicable to proteins, as was shown by the modification of a Dha residue that was chemically introduced into Small Ubiquitin-like Modifier (SUMO).
The introduction of silylated amino acids into biologically active peptides is an attractive approach in medicinal chemistry to overcome some of the poor pharmacological properties associated with such substances.1 The substitution of carbon residues for silicon isosteres hampers the recognition by proteolytic enzymes and thus improves resistance against degradation.2–5 Additionally, silyl groups greatly increase the lipophilicity of peptides, which can be an important factor for enhancing cellular uptake.6
Methods for the silylation of amino acids and peptides have shown to be effective, yet often require harsh conditions or multiple synthetic steps.7–11 Currently, a mild approach that is suitable for the chemical incorporation of silyl groups onto large peptides and proteins is lacking despite the growing popularity of such compounds in medicine.12 Here we report the rapid and selective Cu(II)-catalysed β-silylation of dehydroalanine (Dha) residues in peptides and proteins (Scheme 1).
 | ||
| Scheme 1 Cu(II)-Catalysed β-silylation of Dha (blue) in peptides and proteins using Suginome's reagent (PinBSiMe2Ph). | ||
Dehydroalanines are α,β-unsaturated amino acids13 which occur naturally in ribosomally synthesized and post-translationally modified peptides (RiPPs)14 and can also be introduced chemically into other peptides and proteins.15 They are uniquely reactive as electrophiles, which makes them attractive chemical handles for late-stage modification of such complex natural products.16–27 Cu(II)-Catalyzed β-silyl conjugate additions to α,β-unsaturated carbonyl substrates using PinBSiMe2Ph (also known as Suginome's reagent) have been performed at room temperature, in aqueous media and open to the air.28,29 Therefore, we envisioned this method to be an excellent starting point for exploring the silylation of Dha in peptides and proteins.
First, the β-silylation of Dha acceptors in the thiopeptide thiostrepton was investigated (Fig. 1A). Due to the poor water solubility of thiostrepton 2,2,2-trifluoroethanol (TFE) was used as a co-solvent in our initial screening conditions. Using 10 equivalents of PinBSiMe2Ph and 1 mol% CuSO4, >95% conversion of thiostrepton was achieved within 1 hour, which was accompanied by the rapid formation of the singly and doubly silylated peptide as detected with LC-MS (Fig. 1B). Splitting of the peaks of both the singly and doubly silylated peptide indicated a mixture of diastereomers (Fig. 1B). In contrast to previous studies,29 efficient silylation was achieved without the use of 4-picoline as a Brønsted base. Since the base was not required for the reaction and the presence of amine bases is known to be detrimental for the stability of thiostrepton in solution,30 the base was omitted.
To demonstrate the chemo- and site-selectivity of this approach for the different Dha residues within thiostrepton, the doubly modified products (2a and 2b) were isolated using preparative HPLC (SI-4, ESI†) and characterized via HRMS (SI-5, ESI†) and 2D NMR spectroscopy (SI-6–8, ESI†). It was found that the two terminal Dha residues in the tail region of thiostrepton (Dha16 and Dha17) were modified. Unfortunately, the different diastereomers could not be assigned by 1D and 2D NMR. Finally, the signals of Dha3 and Dhb8 were conserved (Fig. 1C, see SI-6–8, ESI† for a detailed explanation), meaning that our method is not only very fast, but also highly selective.
Next, the scope of the reaction on different Dha-containing peptides was studied, starting with the thiopeptide nosiheptide (Fig. 2A and SI-9, ESI†). Similar conditions were used, except a higher catalyst loading (2 eq.) and addition of 10 equivalents 4-picoline were necessary to achieve efficient silylation. In the analysis of the crude product via LC-MS 80% conversion of nosiheptide to its singly silylated derivative was detected after 1 hour of reaction time (Fig. 2B). Modification of nosiheptide that lacks the terminal Dha, which is present as an impurity in the commercially available nosiheptide, as well as any double modification were not observed. This indicates that, similar to thiostrepton, the reaction is completely selective for the terminal Dha over the internal Dhb.
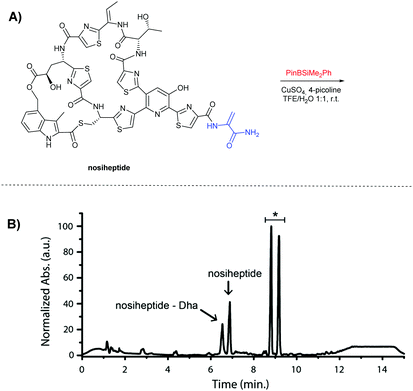 | ||
Fig. 2 (A) β-Silylation of nosiheptide. (B) LC-MS UV (280 nm) of the crude reaction mixture after 1 hour (*![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) single silylation). single silylation). | ||
Nisin Z, a member of the lanthipeptide family of RiPPs, was also investigated as a substrate (Fig. 3A and SI-10, ESI†). In contrast to thiopeptides, nisin Z has a high aqueous solubility, avoiding the need for TFE as a co-solvent and allowing us to conduct the silylation in pure water. It was found that also here the addition of 4-picoline was necessary to achieve efficient silylation. After 1 hour of reaction at room temperature the mixture was analyzed by LC-MS and the mass spectrum of the crude product containing the nisin species was deconvoluted (SI-10, ESI†). A small amount of nisin Z was found, as well as singly and doubly silylated nisin Z products (including the known degradation products of nisin due to water addition (+H2O) and cleavage of the two C-terminal amino acids (–CTerm.))31 (Fig. 3B). In contrast to thiostrepton, in which Dha3 is buried in a macrocycle and Dhb8 does not have carbonyl substituent, the dehydroamino acids in nisin Z are all reactive and accessible resulting in a mixture of regioisomers that could not be resolved using UPLC-MS.
 | ||
Fig. 3 (A) β-Silylation of the lanthipeptide nisin Z. (B) Deconvolution result of the raw mass spectrum of the crude reaction mixture after 1 hour (*![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) single silylation, ** single silylation, **![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) double silylation). double silylation). | ||
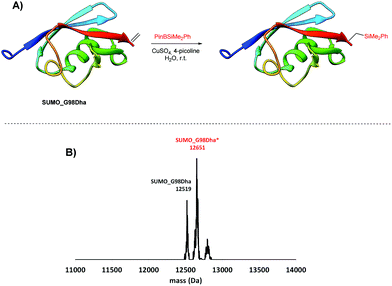
The scope of the reaction was extended to proteins. For this purpose, we performed the reaction on the 12.5 kDa protein Small Ubiquitin-like Modifier (SUMO). SUMO_G98Dha, a mutant with a Dha that was chemically introduced near the C-terminus, was subjected to the silylation conditions (Fig. 4A) and after 1 hour at room temperature the reaction mixture was analysed by LC-MS (SI-11, ESI†). Upon deconvolution of the mass spectra the singly silylated protein was observed, together with a small amount of unreacted SUMO_G98Dha (Fig. 4B).
In conclusion, the Cu(II)-catalysed β-silylation of Dha residues is a straightforward, fast and selective method for the silylation of bioactive peptides and proteins. The reaction is robust and efficient in aqueous media both with and without added co-solvent, enabling the silylation of a variety of natural products. Moreover, the modification of chemically incorporated Dha residues demonstrates that the method is generally applicable in the silylation of peptides and proteins.
The authors wish to thank Roos van Lier for providing SUMO_G98Dha. This project was supported by the Netherlands Organisation for Scientific Research (NWO) (Vici grant 724.013.003). G. R. acknowledges support from the Ministry of Education Culture and Science (Gravitation programme no. 024.001.035).
Conflicts of interest
There are no conflicts to declare.Notes and references
- E. Rémond, C. Martin, J. Martinez and F. Cavelier, Chem. Rev., 2016, 116, 11654–11684 CrossRef PubMed
.
- B. Weidmann, Chimia, 1992, 46, 312–313 CAS
.
- R. Tacke, M. Merget, R. Bertermann, M. Bernd, T. Beckers and T. Reissmann, Organometallics, 2000, 19, 3486–3497 CrossRef CAS
.
- F. Cavelier, D. Marchand, J. Martinez and S. Sagan, J. Pept. Res., 2004, 63, 290–296 CrossRef CAS PubMed
.
- M. Mortensen, R. Husmann, E. Veri and C. Bolm, Chem. Soc. Rev., 2009, 38, 1002–1010 RSC
.
- S. Pujals, J. Fernández-Carneado, M. J. Kogan, J. Martinez, F. Cavelier and E. Giralt, J. Am. Chem. Soc., 2006, 128, 8479–8483 CrossRef CAS PubMed
.
- G. K. Min, D. Hernández and T. Skrydstrup, Acc. Chem. Res., 2013, 46, 457–470 CrossRef CAS PubMed
.
- F. Bartoccini, S. Bartolucci, S. Lucarini and G. Piersanti, Eur. J. Org. Chem., 2015, 3352–3360 CrossRef CAS
.
- J. Y. L. Chung, M. Shevlin, A. Klapars and M. Journet, Org. Lett., 2016, 18, 1812–1815 CrossRef CAS PubMed
.
- Y. J. Liu, Y. H. Liu, Z. Z. Zhang, S. Y. Yan, K. Chen and B. F. Shi, Angew. Chem., Int. Ed., 2016, 55, 13859–13862 CrossRef CAS PubMed
.
- B. B. Zhan, J. Fan, L. Jin and B. F. Shi, ACS Catal., 2019, 9, 3298–3303 CrossRef CAS
.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2016, 79, 629–661 CrossRef CAS PubMed
.
- D. Siodłak, Amino Acids, 2015, 47, 1–17 CrossRef PubMed
.
- P. G. Arnison, M. J. Bibb, G. Bierbaum, A. A. Bowers, T. S. Bugni, G. Bulaj, J. A. Camarero, D. J. Campopiano, G. L. Challis, J. Clardy, P. D. Cotter, D. J. Craik, M. Dawson, E. Dittmann, S. Donadio, P. C. Dorrestein, K. D. Entian, M. A. Fischbach, J. S. Garavelli, U. Göransson, C. W. Gruber, D. H. Haft, T. K. Hemscheidt, C. Hertweck, C. Hill, A. R. Horswill, M. Jaspars, W. L. Kelly, J. P. Klinman, O. P. Kuipers, A. J. Link, W. Liu, M. A. Marahiel, D. A. Mitchell, G. N. Moll, B. S. Moore, R. Müller, S. K. Nair, I. F. Nes, G. E. Norris, B. M. Olivera, H. Onaka, M. L. Patchett, J. Piel, M. J. T. Reaney, S. Rebuffat, R. P. Ross, H. G. Sahl, E. W. Schmidt, M. E. Selsted, K. Severinov, B. Shen, K. Sivonen, L. Smith, T. Stein, R. D. Süssmuth, J. R. Tagg, G. L. Tang, A. W. Truman, J. C. Vederas, C. T. Walsh, J. D. Walton, S. C. Wenzel, J. M. Willey and W. A. Van Der Donk, Nat. Prod. Rep., 2013, 30, 108–160 RSC
.
- J. M. Chalker, S. B. Gunnoo, O. Boutureira, S. C. Gerstberger, M. Fernández-González, G. J. L. Bernardes, L. Griffin, H. Hailu, C. J. Schofield and B. G. Davis, Chem. Sci., 2011, 2, 1666–1676 RSC
.
- J. Dadová, S. R. Galan and B. G. Davis, Curr. Opin. Chem. Biol., 2018, 46, 71–81 CrossRef PubMed
.
- K. Maruyama and M. Kanai, Chem. Lett., 2019, 48, 1421–1432 CrossRef CAS
.
- J.-A. Shin, J. Kim, H. Lee, S. Ha and H.-Y. Lee, J. Org. Chem., 2019, 84, 4558–4565 CrossRef CAS PubMed
.
- R. J. Scamp, E. deRamon, E. K. Paulson, S. J. Miller and J. A. Ellman, Angew. Chem., Int. Ed., 2020, 59, 890–895 CrossRef CAS PubMed
.
- E. A. Hoyt, P. M. S. D. Cal, B. L. Oliveira and G. J. L. Bernardes, Nat. Rev. Chem., 2019, 3, 147–171 CrossRef CAS
.
- J. W. Bogart and A. A. Bowers, Org. Biomol. Chem., 2019, 17, 3653–3669 RSC
.
- M. R. Aronoff, B. Gold and R. T. Raines, Org. Lett., 2016, 18, 1538–1541 CrossRef CAS PubMed
.
- H. M. Key and S. J. Miller, J. Am. Chem. Soc., 2017, 139, 15460–15466 CrossRef CAS PubMed
.
- J. G. Gober, S. V. Ghodge, J. W. Bogart, W. J. Wever, R. R. Watkins, E. M. Brustad and A. A. Bowers, ACS Chem. Biol., 2017, 12, 1726–1731 CrossRef CAS PubMed
.
- A. D. de Bruijn and G. Roelfes, Chem. – Eur. J., 2018, 24, 12728–12733 CrossRef CAS PubMed
.
- A. D. De Bruijn and G. Roelfes, Chem. – Eur. J., 2018, 24, 11314–11318 CrossRef CAS PubMed
.
- R. H. de Vries, J. H. Viel, R. Oudshoorn, O. P. Kuipers and G. Roelfes, Chem. – Eur. J., 2019, 25, 12698–12702 CrossRef CAS PubMed
.
- T. Kitanosono, L. Zhu, C. Liu, P. Xu and S. Kobayashi, J. Am. Chem. Soc., 2015, 137, 15422–15425 CrossRef CAS PubMed
.
- J. A. Calderone and W. L. Santos, Org. Lett., 2012, 14, 2090–2093 CrossRef CAS PubMed
.
- S. Schoof, S. Baumann, B. Ellinger and H. D. Arndt, ChemBioChem, 2009, 10, 242–245 CrossRef CAS PubMed
.
- H. S. Rollema, J. W. Metzger, P. Both, O. P. Kuipers and R. J. Siezen, Eur. J. Biochem., 1996, 241, 716–722 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and data analysis. See DOI: 10.1039/d0cc05026a |
| This journal is © The Royal Society of Chemistry 2020 |