Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
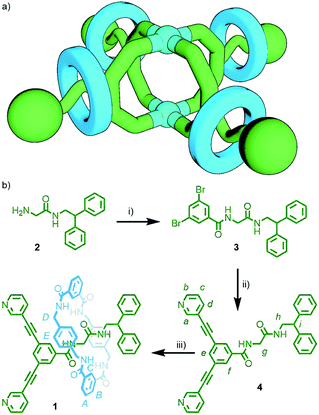
Self-assembly of a porous metallo-[5]rotaxane†
Kevin Kei Gwan
Wong
 ,
Nadia
Hoyas Pérez
,
Andrew J. P.
White
,
Nadia
Hoyas Pérez
,
Andrew J. P.
White
 and
James E. M.
Lewis
and
James E. M.
Lewis
 *
*
Department of Chemistry, Imperial College London, Molecular Sciences Research Hub, 80 Wood Lane, London W12 0BZ, UK. E-mail: james.lewis@imperial.ac.uk
First published on 4th August 2020
Abstract
A mechanically interlocked [2]rotaxane is reported incorporating a ditopic ligand moiety as one of the stoppers. Upon complexation with palladium(II) ions a metallo-[5]rotaxane was formed with a porous Pd2L4 metal–organic cage at the core of the structure. This proof-of-principle system precedes work towards the construction of metal organic polyhedra with switchable mechano-chemical properties.
Metallo-supramolecular self-assembly has been a burgeoning topic over the last few decades, with the synthesis of metal–organic polyhedra (MOPs) of myriad geometries being achieved.1 With the ability to assemble discrete porous architectures in hand, MOPs have been examined for their applications in catalysis,2 drug delivery,3 and biomedicine,4 and used for stabilising reactive species,5 amongst others.
Generally, MOPs are highly symmetrical and ligand components are kept to a minimal level of complexity to avoid detrimental effects on the self-assembly process. However, more intricate assemblies may be desirable for specific applications. As such, in recent years, there has been an interest in developing methods for the construction of functionalised,6 heteroleptic,7 heteronuclear,8 and low symmetry structures.9 In addition there has been increasing activity towards incorporating dynamic10 and stimuli-responsive components into MOPs.11 Of particular note are recent examples by Clever and co-workers of assemblies, derived from photoswitchable dithienylethene-based ligands, capable of undergoing structural changes that modulate the host affinity for anionic guests.12
Mechanically interlocked molecules (MIMs)13 have emerged as a novel class of dynamic ligand. Borne out of interest in the ability of their components to undergo large amplitude relative motion, mechanically interlocked ligands14 have been used to assemble discrete complexes,15 as well as coordination oligomers16 and polymers.17 Indeed, Schurko, Loeb and co-workers have demonstrated both pirouetting18 and shuttling19 of macrocycles within metal–organic rotaxane frameworks (MORFs)20 in the solid state, and Hupp, Farha, Stoddart and co-workers have reported the operation of an electrochemically switchable catenane residing in the pores of a MOF.21 Despite these exciting preliminary results, self-assembly with dynamic ligands is still a nascent area of research.22
The stimuli-responsive motion of MIMs has been exploited to control the ingress and egress of chemical payloads into and out of the pores of silica nanoparticles through changing the proximity of the steric bulk of an interlocked macrocycle relative to the pore opening.23 This concept could in theory be translated to MOPs, allowing occlusion of portals, thus inhibiting guest movement between the central cavity and external environment in a controlled manner. The modulation of steric bulk around the cage periphery could also offer the potential for allosteric regulation of catalysis.24
MOPs have been prepared with ligands that allow for the formation of dynamic libraries of metallo[n]catenanes,25 and a mixed-metal cluster threaded with a ligand was recently shown to assemble into a capsular [13]rotaxane structure.26 However, to the best of our knowledge, there have been no reports on the use of organic MIMs as ligands for the self-assembly of metal–organic cages – three-dimensional, porous MOPs. In this work we report a proof-of-principle system in which a dipyridyl [2]rotaxane ligand is shown to self-assemble in the presence of palladium(II) ions to form a Pd2L4 architecture in which the exohedral faces are decorated with four interlocked macrocycles; namely, a metallo-[5]rotaxane. These results validate principles of ongoing research towards the preparation of MOPs derived from interlocked ligands in which the proximity of sub-components can be manipulated in a stimuli-responsive manner, allowing control of payload exchange between the internal cage cavity and the external environment.
[2]Rotaxane 1 (Scheme 1) was designed incorporating one diphenylmethane and one m-bis(pyridin-3-ylethynyl)phenyl stopper. The former simply acts as a physical barrier to prevent dethreading of the macrocycle, whilst the latter is a motif known to assemble in the presence of square planar palladium(II) ions to form quadruply-stranded dinuclear cage architectures,27 with cavities capable of binding guests through hydrogen bonding interactions.28 Provided the axle and macrocycle components do not interfere with the self-assembly of this ligand moiety, addition of palladium(II) ions to 1 would be expected to yield a metallo-[5]rotaxane with a porous core (Scheme 1a).
A straightforward synthetic route to 1 was identified (Scheme 1b). Precursor 2 was prepared from Boc-glycine and 2,2-diphenylethylamine according to literature procedure.29 Subsequent amide condensation with 3,5-dibromobenzoic acid (77%), following by Sonogashira coupling with 3-ethynylpyridine (75%), gave non-interlocked axle 4 in 58% yield over the two steps (see ESI† for details).
It was deemed prudent to examine binding of palladium(II) ions with the axle component by itself initially. To this end, 4 was combined with 0.5 equivalents of [Pd(CH3CN)4](BF4)2 in d6-DMSO. Immediate (<10 minutes) formation of a new species was observed by 1H NMR spectroscopy (Fig. 1b), with significant downfield shifts of the protons adjacent to the pyridyl nitrogen atoms (Ha and Hb, Δδ = 0.80 and 0.77 ppm, respectively) indicating coordination of the palladium(II) ions. From DOSY NMR (Fig. 1c and Table 1) the diffusion coefficient, D, of the complex (7.76 × 10−11 m2 s−1) was found to be approximately half that of the ligand (1.72 × 10−10 m2 s−1), congruous with previous reports of Pd2L4 cages, and gave a calculated hydrodynamic radius (RH) consistent with the optimised structure of the Pd2L4 ‘paddle-wheel’ assembly (13.0 Å; see ESI†). Finally, isotopic patterns consistent with this architecture were observed by mass spectrometry (MS; Fig. S25–S27, ESI†).30
 | ||
| Fig. 1 1H NMR spectra (500 MHz, d6-DMSO, 298 K) of (a) 4, and (b) [Pd2(4)4](BF4)4, and (c) DOSY NMR spectrum (500 MHz, d6-DMSO, 298 K) of [Pd2(4)4](BF4)4. For axle labelling see Scheme 1. | ||
| Compound | D (m2 s−1) | R H (Å) | D ligand/cage ratio |
|---|---|---|---|
| 4 | 1.72 × 10−10 | 5.9 | 2.2 |
| [Pd2(4)4](BF4)4 | 7.76 × 10−11 | 13.0 | |
| 1 | 1.26 × 10−10 | 8.0 | 1.9 |
| [Pd2(1)4](BF4)4 | 6.81 × 10−11 | 14.8 |
Having confirmed that the axle did not interfere with the self-assembly of the ligand stopper unit, [2]rotaxane 1 was subsequently prepared. The rotaxane ligand was synthesised under pseudo-high dilution conditions through simultaneous addition of solutions of isophthaloyl dichloride and p-xylylenediamine to a solution of the axle, 4, and NEt3 in anhydrous CHCl3, resulting in clipping of the tetralactam macrocycle around the glycylglycine motif,31 giving 1 in 10% isolated yield (the low yield is chiefly ascribed to the proximity of the bulky dipyridine stopper to the template motif).
The identity and interlocked nature of the ligand was confirmed by MS (m/z = 1115 [M + Na]+) and NMR spectroscopy (Fig. 2b). In comparison to the free axle (Fig. 2a) most signals of the dipyridyl stopper were minimally perturbed (Δδ ≤ 0.1 ppm). However, Hf, directed towards the glycylglycine unit, was shifted dramatically upfield (Δδ = 0.38 ppm), as was Hg, the methylene unit upon which the macrocycle was expected to reside (Δδ = 1.39). Additionally, signals for both the axle and macrocycle components were observed to diffuse at the same rate by DOSY NMR (Fig. S17, ESI†).
 | ||
| Fig. 2 1H NMR spectra (400 MHz, CDCl3, 298 K) of (a) axle 4, and (b) [2]rotaxane 1. For axle and macrocycle labelling see Scheme 1. (c) SCXRD structure of 1 shown as space-fill model, and (d) abbreviated structure showing intercomponent H-bonding interactions (hydrogen atoms not involved in intramolecular H-bonding omitted for clarity). Distances (Å) and angles (°): O1⋯H 1.92, O1⋯H–N 157; O2⋯H 2.04, O2⋯H–N 170; O3⋯H 1.91, O3⋯H–N 173; O4⋯H 1.94, O4⋯H–N 174. | ||
Ultimately the mechanically interlocked structure of 1 was confirmed in the solid state by single crystal X-ray diffraction (SCXRD; Fig. 2c). The SCXRD structure of 1 showed two of the macrocyclic carbonyl groups to be directed endohedrally, the other two exohedrally, to give four intramolecular, intercomponent N–H⋯carbonyl hydrogen bonding interactions between the macrocycle and axle (Fig. 2d; N–H⋯O distances 1.91–2.04 Å), a motif previously observed in the solid state structures of similar systems.29,32
 | ||
| Fig. 3 1H NMR spectra (500 MHz, d6-DMSO, 298 K) of (a) [2]rotaxane 1, and (b) metallo-[5]rotaxane [Pd2(1)4](BF4)4. (c) DOSY NMR spectrum (500 MHz, d6-DMSO, 298 K) of [Pd2(1)4](BF4)4. For axle and macrocycle labelling see Scheme 1. Geometry optimised structure (PM6) of [Pd2(1)4]4+ shown (d) down the Pd–Pd axis, and (e) from the side. | ||
With the rotaxane in hand the self-assembly of the interlocked ligand with palladium(II) was examined. Pleasingly, addition of [Pd(CH3CN)4](BF4)2 to rotaxane 1 in d6-DMSO resulted in similar behaviour to the non-interlocked axle. A single set of signals was observed in the 1H NMR spectrum (Fig. 3b), with downfield shifts (Δδ = 0.78 and 0.73 ppm for Ha and Hb, respectively) of the pyridyl signals relative to the free ligand (Fig. 3a), indicating coordination to the palladium(II) ions. A diffusion coefficient of 6.81 × 10−11 m2 s−1 (Fig. 3c and Table 1) was observed by DOSY NMR, approximately half that of the ligand (1.26 × 10−10 m2 s−1), corresponding to an RH of 14.8 Å, consistent with the optimised structure of the assembly (Fig. 3d and e). With further support from MS (m/z = 1542 {[Pd2(1)4](A−)}3+),30 the successful formation of the desired Pd2L4, metallo-[5]rotaxane was concluded. Although the use of DMSO as solvent precluded the use of variable temperature NMR experiments to determine a rate constant, the observation of a single set of signals for the macrocycle component (Fig. 3b) indicated that in the metallo-[5]rotaxane structure these remain sufficiently unencumbered to continue pirouetting about the axles at a fast rate compared to the NMR timescale.
In summary we have reported the synthesis of a [2]rotaxane ligand capable of self-assembly with palladium(II) ions to form a porous metallo-supramolecular cage with exohedral rotaxane units, i.e. a metallo-[5]rotaxane. This proof-of-principle system highlights the potential for incorporating dynamic mechanically interlocked components into MOPs. In this current model system the macrocycle components are dynamic in terms of rotational motion (pirouetting), but static with respect to linear motion, i.e. shuttling, along the axle. Work in our lab is now ongoing to prepare ligands incorporating stimuli-responsive mechanically interlocked moieties into the framework, offering the potential for triggered occlusion of cage portals. In this manner it is hoped the kinetics of guest exchange with the host cavity may be modulated in a controllable manner.33
This work was supported by an Imperial College Research Fellowship (JEML) and a Royal Society Research Grant (RG170321). We thank Peter Haycock for assistance with NMR data collection, Dr Lisa Haigh for MS, and Prof. Matthew Fuchter for useful discussions and access to resources.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) M. Fujita, Chem. Soc. Rev., 1998, 27, 417–425 RSC; (b) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853–907 CrossRef CAS PubMed; (c) B. J. Holliday and C. A. Mirkin, Angew. Chem., Int. Ed., 2001, 40, 2022–2043 CrossRef CAS; (d) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed; (e) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed.
- (a) M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS PubMed; (b) L. J. Jongkind, X. Caumes, A. P. T. Hartendorp and J. N. H. Reek, Acc. Chem. Res., 2018, 51, 2115–2128 CrossRef CAS PubMed; (c) I. Sinha and P. S. Mukherjee, Inorg. Chem., 2018, 57, 4205–4221 CrossRef CAS PubMed; (d) C. Tan, D. Chu, X. Tang, Y. Liu, W. Xuan and Y. Cui, Chem. – Eur. J., 2019, 25, 662–672 CrossRef CAS PubMed; (e) Y. Fang, J. A. Powell, E. Li, Q. Wang, Z. Perry, A. Kirchon, X. Yang, Z. Xiao, C. Zhu, L. Zhang, F. Huang and H. C. Zhou, Chem. Soc. Rev., 2019, 48, 4707–4730 RSC; (f) R. J. Severinsen, G. J. Rowlands and P. G. Plieger, J. Inclusion Phenom. Macrocyclic Chem., 2020, 96, 29–42 CAS.
- (a) B. Therrien, G. Süss-Fink, P. Govindaswamy, A. K. Renfrew and P. J. Dyson, Angew. Chem., Int. Ed., 2008, 47, 3773–3776 CrossRef CAS PubMed; (b) J. E. M. Lewis, E. L. Gavey, S. A. Cameron and J. D. Crowley, Chem. Sci., 2012, 3, 778–784 RSC.
- (a) T. R. Cook, V. Vajpayee, M. H. Lee, P. J. Stang and K. W. Chi, Acc. Chem. Res., 2013, 46, 2464–2474 CrossRef CAS PubMed; (b) A. Casini, B. Woods and M. Wenzel, Inorg. Chem., 2017, 56, 14715–14729 CrossRef CAS; (c) H. Sepehrpour, W. Fu, Y. Sun and P. J. Stang, J. Am. Chem. Soc., 2019, 141, 14005–14020 CrossRef CAS.
- (a) P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697–1699 CrossRef CAS PubMed; (b) M. Yamashina, Y. Sei, M. Akita and M. Yoshizawa, Nat. Commun., 2014, 5, 4662 CrossRef CAS PubMed; (c) K. Niki, T. Tsutsui, M. Yamashina, M. Akita and M. Yoshizawa, Angew. Chem., Int. Ed., 2020, 59, 10489–10492 CrossRef CAS PubMed.
- (a) M. Tominaga, K. Suzuki, T. Murase and M. Fujita, J. Am. Chem. Soc., 2005, 127, 11950–11951 CrossRef CAS PubMed; (b) N. Kamiya, M. Tominaga, S. Sato and M. Fujita, J. Am. Chem. Soc., 2007, 129, 3816–3817 CrossRef CAS PubMed; (c) J. E. M. Lewis, C. J. McAdam, M. G. Gardiner and J. D. Crowley, Chem. Commun., 2013, 49, 3398–3400 RSC; (d) J. E. M. Lewis, A. B. S. Elliott, C. J. McAdam, K. C. Gordon and J. D. Crowley, Chem. Sci., 2014, 5, 1833–1843 RSC.
- (a) W. M. Bloch and G. H. Clever, Chem. Commun., 2017, 53, 8506–8516 RSC; (b) D. Bardhan and D. K. Chand, Chem. – Eur. J., 2019, 25, 12241–12269 CrossRef CAS PubMed.
- (a) L. Li, D. J. Fanna, N. D. Shepherd, L. F. Lindoy and F. Li, J. Inclusion Phenom. Macrocyclic Chem., 2015, 82, 3–12 CrossRef CAS; (b) H. Li, Z. J. Yao, D. Liu and G. X. Jin, Coord. Chem. Rev., 2015, 293–294, 139–157 CrossRef CAS; (c) M. Hardy and A. Lützen, Chem. – Eur. J., 2020 DOI:10.1002/chem.202001602.
- (a) J. E. M. Lewis and J. D. Crowley, ChemPlusChem, 2020, 85, 815–827 CrossRef CAS PubMed; (b) S. Hiraoka and M. Fujita, J. Am. Chem. Soc., 1999, 121, 10239–10240 CrossRef CAS; (c) S. K. Sen and R. Natarajan, Inorg. Chem., 2019, 58, 7180–7188 CrossRef CAS PubMed; (d) D. Ogata and J. Yuasa, Angew. Chem., Int. Ed., 2019, 58, 18424–18428 CrossRef CAS PubMed; (e) J. E. M. Lewis, A. Tarzia, A. J. P. White and K. E. Jelfs, Chem. Sci., 2020, 11, 677–683 RSC; (f) L. S. Lisboa, J. A. Findlay, L. J. Wright, C. G. Hartinger and J. D. Crowley, Angew. Chem., Int. Ed., 2020, 59, 11101–11107 CrossRef CAS PubMed.
- (a) M. Krick, J. Holstein, C. Würtele and G. H. Clever, Chem. Commun., 2016, 52, 10411–10414 RSC; (b) S. Löffler, J. Lübben, A. Wuttke, R. A. Mata, M. John, B. Dittrich and G. H. Clever, Chem. Sci., 2016, 7, 4676–4684 RSC.
- (a) M. Han, Y. Luo, B. Damaschke, L. Gómez, X. Ribas, A. Jose, P. Peretzki, M. Seibt and G. H. Clever, Angew. Chem., Int. Ed., 2016, 55, 445–449 CrossRef CAS PubMed; (b) S. Oldknow, D. R. Martir, V. E. Pritchard, M. A. Blitz, C. W. G. Fishwick, E. Zysman-Colman and M. J. Hardie, Chem. Sci., 2018, 9, 8150–8159 RSC; (c) Y. Gu, E. A. Alt, H. Wang, X. Li, A. P. Willard and J. A. Johnson, Nature, 2018, 560, 65–69 CrossRef CAS PubMed.
- (a) M. Han, R. Michel, B. He, Y. S. Chen, D. Stalke, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2013, 52, 1319–1323 CrossRef CAS PubMed; (b) R. J. Li, J. J. Holstein, W. G. Hiller, J. Andréasson and G. H. Clever, J. Am. Chem. Soc., 2019, 141, 2097–2103 CrossRef CAS PubMed.
- C. J. Bruns and J. F. Stoddart, The Nature of the Mechanical Bond, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2016 Search PubMed.
- J. E. M. Lewis, M. Galli and S. M. Goldup, Chem. Commun., 2017, 53, 298–312 RSC.
- (a) G. J. E. Davidson and S. J. Loeb, Dalton Trans., 2003, 3, 4319–4323 RSC; (b) A. Noor, W. K. C. Lo, S. C. Moratti and J. D. Crowley, Chem. Commun., 2014, 50, 7044–7047 RSC; (c) E. Viljoen, K. Zhu and S. J. Loeb, Chem. – Eur. J., 2016, 22, 7479–7484 CrossRef CAS PubMed.
- (a) C. Hamers, O. Kocian, F. M. Raymo and J. F. Stoddart, Adv. Mater., 1998, 10, 1366–1369 CrossRef CAS; (b) A. Noor, S. C. Moratti and J. D. Crowley, Chem. Sci., 2014, 5, 4283–4290 RSC.
- (a) V. N. Vukotic and S. J. Loeb, Chem. Soc. Rev., 2012, 41, 5896–5906 RSC; (b) L. Gao, Z. Zhang, B. Zheng and F. Huang, Polym. Chem., 2014, 5, 5734–5739 RSC; (c) Y. Shi, Z. Yang, H. Liu, Z. Li, Y. Tian and F. Wang, ACS Macro Lett., 2015, 4, 6–10 CrossRef CAS; (d) J. E. M. Lewis, Org. Biomol. Chem., 2019, 17, 2442–2447 RSC.
- V. N. Vukotic, K. J. Harris, K. Zhu, R. W. Schurko and S. J. Loeb, Nat. Chem., 2012, 4, 456–460 CrossRef CAS PubMed.
- K. Zhu, C. A. O’Keefe, V. N. Vukotic, R. W. Schurko and S. J. Loeb, Nat. Chem., 2015, 7, 514–519 CrossRef CAS PubMed.
- S. J. Loeb, Chem. Commun., 2005, 1511–1518 RSC.
- Q. Chen, J. Sun, P. Li, I. Hod, P. Z. Moghadam, Z. S. Kean, R. Q. Snurr, J. T. Hupp, O. K. Farha and J. F. Stoddart, J. Am. Chem. Soc., 2016, 138, 14242–14245 CrossRef CAS PubMed.
- H. Deng, M. A. Olsen, J. F. Stoddart and O. M. Yaghi, Nat. Chem., 2010, 2, 439–443 CrossRef CAS PubMed.
- M. W. Ambrogio, C. R. Thomas, Y. L. Zhao, J. I. Zink and J. F. Stoddart, Acc. Chem. Res., 2011, 44, 903–913 CrossRef CAS PubMed.
- V. Martí-Centelles, R. L. Spicer and P. J. Lusby, Chem. Sci., 2020, 11, 3236–3240 RSC.
- (a) S. P. Black, A. R. Stefankiewicz, M. M. J. Smulders, D. Sattler, C. A. Schalley, J. R. Nitschke and J. K. M. Sanders, Angew. Chem., Int. Ed., 2013, 52, 5749–5752 CrossRef CAS PubMed; (b) S. P. Black, D. M. Wood, F. B. Schwarz, T. K. Ronson, J. J. Holstein, A. R. Stefankiewicz, C. A. Schalley, J. K. M. Sanders and J. R. Nitschke, Chem. Sci., 2016, 7, 2614–2620 RSC.
- J. Ferrando-Soria, A. Fernandez, D. Asthana, S. Nawaz, I. J. Vitorica-Yrezabal, G. F. S. Whitehead, C. A. Muryn, F. Tuna, G. A. Timco, N. D. Burton and R. E. P. Winpenny, Nat. Commun., 2019, 10, 3720 CrossRef PubMed.
- P. Liao, B. W. Langloss, A. M. Johnson, E. R. Knudsen, F. S. Tham, R. R. Julian and R. J. Hooley, Chem. Commun., 2010, 46, 4932–4934 RSC.
- D. P. August, G. S. Nichol and P. J. Lusby, Angew. Chem., Int. Ed., 2016, 55, 15022–15026 CrossRef CAS PubMed.
- D. S. Marlin, D. G. Cabrera, D. A. Leigh and A. M. Z. Slawin, Angew. Chem., Int. Ed., 2006, 45, 77–83 CrossRef CAS PubMed.
- Despite preparing the BF4− salts of the cages, under the MS conditions the dominant peaks corresponded to HCO2− adducts. However, the isotopic patterns observed were consistent with the cationic Pd2L4 assemblies.
- D. A. Leigh, A. Murphy, J. P. Smart and A. M. Z. Slawin, Angew. Chem., Int. Ed. Engl., 1997, 36, 728–732 CrossRef CAS.
- D. M. D’Souza, D. A. Leigh, L. Mottier, K. M. Mullen, F. Paolucci, S. J. Teat and S. Zhang, J. Am. Chem. Soc., 2010, 132, 9465–9470 CrossRef PubMed.
- T. Y. Kim, R. A. S. Vasdev, D. Preston and J. D. Crowley, Chem. – Eur. J., 2018, 24, 14878–14890 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, spectroscopic data, NMR spectra, crystallographic details and computational methods. CCDC 2001103. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc04780e |
| This journal is © The Royal Society of Chemistry 2020 |