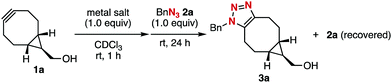Open Access Article
Open Access ArticleSelective strain-promoted azide–alkyne cycloadditions through transient protection of bicyclo[6.1.0]nonynes with silver or gold†
Keisuke
Adachi
a,
Tomohiro
Meguro
a,
Yuki
Sakata
a,
Kazunobu
Igawa
 b,
Katsuhiko
Tomooka
b,
Katsuhiko
Tomooka
 b,
Takamitsu
Hosoya
b,
Takamitsu
Hosoya
 a and
Suguru
Yoshida
a and
Suguru
Yoshida
 *a
*a
aLaboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University (TMDU), 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan. E-mail: s-yoshida.cb@tmd.ac.jp
bInstitute for Materials Chemistry and Engineering, Kyushu University, 6-1 Kasuga-koen, Kasuga, Fukuoka 816-8580, Japan
First published on 16th July 2020
Abstract
Complexation of bicyclo[6.1.0]nonynes with a cationic silver or gold salt results in protection from a click reaction with azides. The cycloalkyne protection using the silver or gold salt enables selective strain-promoted azide–alkyne cycloadditions of diynes keeping the bicyclo[6.1.0]nonyne moiety unreacted.
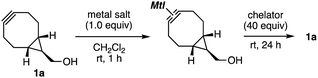
Click reactions, such as strain-promoted azide–alkyne cycloaddition (SPAAC) using cycloalkynes, have been used for reliable molecular conjugation in a broad range of research fields including materials chemistry, pharmaceutical sciences, and chemical biology.1–7 In particular, remarkable reactivities of bicyclo[6.1.0]nonynes (BCNs) realized catalyst-free functionalizations by reaction with a number of ynophiles such as azides, nitrones, nitrile oxides, tetrazines, triazines, sydnones, thiophene S,S-dioxides, and so on.5 In the course of our studies on click chemistry,6 we recently developed a transient protection method for cycloalkynes involving BCNs from the SPAAC reaction by complexation with (MeCN)4CuBF4, which was easily deprotected by treatment with chelators (Fig. 1A).7 A wide variety of functionalized cycloalkynes were synthesized using an azide-to-cycloalkyne switching approach by the protection of a cycloalkyne having a terminal alkyne moiety with (MeCN)4CuBF4 followed by copper-catalyzed azide–alkyne cycloaddition (CuAAC) with functionalized azides and subsequent deprotection with an aqueous solution of disodium ethylenediaminetetraacetate (EDTA·2Na) (Fig. 1B).8 We herein disclose a selective protection method for BCNs from other cycloalkynes by silver or gold complexation, realizing facile synthesis of functionalized BCNs by selective SPAAC reactions using diyne platforms leaving the BCN moiety intact (Fig. 1C).
 | ||
| Fig. 1 Protection methods of cycloalkynes. (A) Protection with copper. (B) The azide-to-cycloalkyne switching approach. (C) This work. FG = functional group. | ||
With previous reports of cyclooctyne–metal complexes in mind,9,10 we envisioned that silver and gold salts can protect cycloalkynes from the SPAAC reaction by complexation. Thus, we screened silver and gold salts in the complexation with BCN 1a in CDCl3 followed by the addition of azide 2a (Table 1). As a result, a variety of silver salts decreased the yield of triazole 3a along with the recovery of azide 2a and precipitate formation of 1a–metal complexes (entries 2–7), while azide 2a was completely consumed when the reaction was performed without any metal salt (entry 1). In particular, the examination using AgBF4 resulted in no triazole formation and almost complete recovery of azide 2a (entry 7), clearly showing that the cationic silver salt completely prevented the SPAAC reaction. Furthermore, AuBF4 prepared from AuCl and AgBF4 also realized the protection of cycloalkyne 1a from the SPAAC reaction (entry 8).
Deprotection of BCN–metal complexes 1a–AgBF4 and 1a–AuBF4 was achieved by proper choice of the silver and gold salts, and chelators (Table 2). For example, the treatment of 1a–AgBF4 with an aqueous solution of ammonia or EDTA·2Na resulted in the recovery of 1a in low to moderate yields along with the decomposition of cycloalkyne 1a (entries 1 and 2). In sharp contrast, the deprotection of 1a–AgBF4 with a solid-phase metal scavenger SiliaMetS Thiourea proceeded efficiently (entry 3). In particular, increasing the amount of SiliaMetS Thiourea improved the recovery yield (entry 4). The deprotection of 1a–AgBF4 with a polystyrene-conjugated phosphine also took place smoothly (entry 5). Among a variety of conditions screened for the deprotection of 1a–AuBF4 (entries 6–10), we succeeded in the efficient deprotection with SiliaMetS Thiourea in THF (entry 9).
| Entry | Metal salt | Chelator | 1a (%) |
|---|---|---|---|
| a Yields were determined using 1H NMR analysis. b Chelators (80 equiv.) were used. c AuBF4 was prepared from AuCl and AgBF4. d THF was used instead of CH2Cl2. | |||
| 1 | AgBF4 | 15 M aq. NH3 | 55 |
| 2 | AgBF4 | 0.1 M aq. EDTA·2Na | 15 |
| 3 | AgBF4 | SiliaMetS Thiourea | 69 |
| 4b | AgBF4 | SiliaMetS Thiourea | 93 |
| 5b | AgBF4 | Resin(polystyrene)-PPh2 (PS-TPP) | 82 |
| 6c | AuBF4 | 15 M aq. NH3 | 0 |
| 7c | AuBF4 | 0.1 M aq. EDTA·2Na | 24 |
| 8b,c | AuBF4 | SiliaMetS Thiourea | 58 |
| 9b,c,d | AuBF4 | SiliaMetS Thiourea | 83 |
| 10b,c | AuBF4 | Resin(polystyrene)-PPh2 (PS-TPP) | 0 |
Attempts to protect dibenzo-fused cyclooctyne (DIBO) 1b11 and 4,8-diazacyclononyne (DACN) 1c12 with silver or gold revealed their decreasing coordination strength compared to the complex between BCN 1a and silver or gold (Table 3). When (MeCN)4CuBF4 was used, cycloalkynes 1b and 1c were successfully protected resulting in the recovery of azide 2a in excellent yields (entries 1 and 4). On the other hand, the treatment of cycloalkynes 1b and 1c with AgBF4 or AuBF4 in CDCl3 followed by the addition of azide 2a furnished triazoles 3b and 3c in low to high yields (entries 2, 3, 5, and 6). Of note, the coordination strength between DACN 1c and silver or gold was significantly weak, leading to the recovery of azide 2a in low yields. These results clearly show the contrasting differences in the complexation of cycloalkynes with copper, silver, and gold depending on the electronic nature and ring strain of cycloalkynes.
Complexation of BCN 1a with AgBF4 served as protection from [2+3] cycloaddition with nitrone 4 (Table 4). While BCN 1a smoothly reacted with nitrone 4 without the protection providing dihydroisoxazole 5 in high yield (entry 1), complexation of BCN 1a with transition metals prevented the cycloalkyne–nitrone cycloaddition (entries 2–4).13 However, nitrone 4 was consumed completely by decomposition when using (MeCN)4CuBF4 or AuBF4 (entries 2 and 4). On the other hand, the complexation of 1a with AgBF4 realized the protection of BCN 1a along with the recovery of nitrone 4 in good yield (entry 3).
Complexation with silver or gold realized the selective protection of BCN in the presence of other cycloalkynes (Table 5). An equimolar mixture between BCN 1a and DIBO 1b (1.0 equiv. each) smoothly reacted with benzyl azide (2a) (1.0 equiv.) without protection to furnish a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 mixture of triazoles 3a and 3b (entry 1). The pretreatment of cycloalkynes 1a and 1b (1.0 equiv. each) with 1.0 equiv. of metal salts drastically changed the ratio of 3a to 3b (entries 2–4). In particular, we succeeded in the selective SPAAC reaction of DIBO 1b affording triazole 3b in high yield when using AuBF4 by virtue of the BCN-selective protection (entry 4). The SPAAC reaction of an equimolar mixture of BCN 1a and DACN 1c with azide 2a also proceeded efficiently to provide a ca. 1.6
1.2 mixture of triazoles 3a and 3b (entry 1). The pretreatment of cycloalkynes 1a and 1b (1.0 equiv. each) with 1.0 equiv. of metal salts drastically changed the ratio of 3a to 3b (entries 2–4). In particular, we succeeded in the selective SPAAC reaction of DIBO 1b affording triazole 3b in high yield when using AuBF4 by virtue of the BCN-selective protection (entry 4). The SPAAC reaction of an equimolar mixture of BCN 1a and DACN 1c with azide 2a also proceeded efficiently to provide a ca. 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of triazoles 3a and 3c (entry 5). The pretreatment of cycloalkynes 1a and 1c with metal salts prevented the formation of triazole 3a (entries 6–8). In particular, treatment of cycloalkynes 1a and 1c (1.0 equiv. each) with AgBF4 (1.0 equiv.) followed by the addition of azide 2a exclusively furnished triazole 3c in excellent yield (entry 7).
1 mixture of triazoles 3a and 3c (entry 5). The pretreatment of cycloalkynes 1a and 1c with metal salts prevented the formation of triazole 3a (entries 6–8). In particular, treatment of cycloalkynes 1a and 1c (1.0 equiv. each) with AgBF4 (1.0 equiv.) followed by the addition of azide 2a exclusively furnished triazole 3c in excellent yield (entry 7).
| Entry | 1b or 1c | Metal salt | 3a (%) | 3b or 3ca (%) | 2a (%) |
|---|---|---|---|---|---|
| a Yields were determined using 1H NMR analysis. b AuBF4 was prepared from AuCl and AgBF4. | |||||
| 1 | 1b | None | 43 | 3b, 53 | 0 |
| 2 | 1b | (MeCN)4CuBF4 | 5 | 3b, 58 | 36 |
| 3 | 1b | AgBF4 | 17 | 3b, 74 | 0 |
| 4b | 1b | AuBF4 | 0 | 3b, 92 | 1 |
| 5 | 1c | None | 60 | 3c, 37 | 0 |
| 6 | 1c | (MeCN)4CuBF4 | 0 | 3c, 68 | 28 |
| 7 | 1c | AgBF4 | 0 | 3c, 99 | 0 |
| 8b | 1c | AuBF4 | 0 | 3c, 74 | 18 |
The synthetic utility of the DIBO- and DACN-selective SPAAC reactions in the presence of a BCN moiety through the complexation was showcased by the selective triazole formation of diynes 6 and 8 keeping the BCN moiety unreacted (Fig. 2). Indeed, the pretreatment of diyne 6 with AuBF4 followed by the addition of azide 2b and subsequent removal of the gold salt with SiliaMetS Thiourea provided triazole 7 in good yield by the SPAAC reaction at the DIBO moiety without reacting the BCN moiety (Fig. 2A). Furthermore, we also achieved the DACN-selective triazole formation of diyne 8 by complexation with AgBF4, addition of azide 2b, and the removal of the silver salt with SiliaMetS Thiourea (Fig. 2B). Since the remaining BCN moiety contributes significantly to the catalyst-free click conjugation with various ynophiles in materials chemistry and chemical biology, the DIBO- and DACN-selective triazole formation of diynes allowed for sequential conjugations of a broad range of functional molecules.5
 | ||
| Fig. 2 Selective SPAAC reactions of diynes 6 and 8. (A) Selective reaction of 6. (B) Selective reaction of 8. | ||
In conclusion, we have developed an efficient method for the transient protection of BCNs by complexation with silver or gold, enabling DIBO- or DACN-selective triazole formation.14 The selective SPAAC reactions realized the preparation of functionalized BCNs by the selective click conjugation of diynes. Further studies of cycloalkyne–metal complexes involving detailed solvent effects, protection from various ynophiles, and applications of sequential triazole formations of diynes are now in progress.
This work was supported by JSPS KAKENHI Grant Numbers JP19K05451 (C; S. Y.), JP18J11113 (JSPS Research Fellow; T. M.), JP18H02104 (B; T. H.), and JP18H04386 (Middle Molecular Strategy; T. H.); the Naito Foundation (S. Y.); the Japan Agency for Medical Research and Development (AMED) under Grant Number JP20am0101098 (Platform Project for Supporting Drug Discovery and Life Science Research, BINDS); the Cooperative Research Project of Research Center for Biomedical Engineering; and the Research Program of “Five-Star Alliance” in “NJRC Mater. & Dev.”
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS
; (b) C. S. McKay and M. G. Finn, Chem. Biol., 2014, 21, 1075 CrossRef CAS
; (c) J. Lahann, Click Chemistry for Biotechnology and Materials Science, John Wiley & Sons, West Sussex, 2009 CrossRef
.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed
.
-
(a) M. F. Debets, C. W. J. van der Doelen, F. P. J. T. Rutjes and F. L. van Delft, ChemBioChem, 2010, 11, 1168 CrossRef CAS
; (b) J. C. Jewett and C. R. Bertozzi, Chem. Soc. Rev., 2010, 39, 1272 RSC
; (c) E. M. Sletten and C. R. Bertozzi, Acc. Chem. Res., 2011, 44, 666 CrossRef CAS PubMed
; (d) S. Arumugam, S. V. Orski, N. E. Mbua, C. McNitt, G.-J. Boons, J. Locklin and V. V. Popik, Pure Appl. Chem., 2013, 85, 1499 CAS
; (e) J. Dommerholt, F. P. J. T. Rutjes and F. L. van Delft, Top. Curr. Chem., 2016, 374, 16 CrossRef
.
-
(a) A.-C. Knall and C. Slugovc, Chem. Soc. Rev., 2013, 42, 5131 RSC
; (b) Z.-J. Zheng, D. Wang, Z. Xu and L.-W. Xu, Beilstein J. Org. Chem., 2015, 11, 2557 CrossRef CAS
; (c) S. Yoshida, Bull. Chem. Soc. Jpn., 2018, 91, 1293 CrossRef CAS
; (d) S. Yoshida, Org. Biomol. Chem., 2020, 18, 1550 RSC
.
-
(a) J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422 CrossRef CAS
; (b) A. M. Jawalekar, E. Reubsaet, F. P. J. T. Rutjes and F. L. van Delft, Chem. Commun., 2011, 47, 3198 RSC
; W. Chen, D. Wang, C. Dai, D. Hamelberg and B. Wang, Chem. Commun., 2012, 48, 1736 Search PubMed
; (c) T. Cruchter, K. Harms and E. Meggers, Chem. – Eur. J., 2013, 19, 16682 CrossRef CAS PubMed
; (d) D. Wang, W. Chen, Y. Zheng, C. Dai, K. Wang, B. Ke and B. Wang, Org. Biomol. Chem., 2014, 12, 3950 RSC
; (e) S. Wallace and J. W. Chin, Chem. Sci., 2014, 5, 1742 RSC
; (f) L. Plougastel, O. Koniev, S. Specklin, E. Decuypere, C. Créminon, D.-A. Buisson, A. Wagner, S. Kolodych and F. Taran, Chem. Commun., 2014, 50, 9376 RSC
; (g) D. Wang, E. Viennois, K. Ji, K. Damera, A. Draganov, Y. Zheng, C. Dai, D. Merlin and B. Wang, Chem. Commun., 2014, 50, 15890 RSC
; (h) J. Dommerholt, O. van Rooijen, A. Borrmann, C. F. Guerra, F. M. Bickelhaupt and F. L. van Delft, Nat. Commun., 2014, 5, 5378 CrossRef CAS
; (i) T. H. Poole, J. A. Reisz, W. Zhao, L. B. Poole, C. M. Furdui and S. B. King, J. Am. Chem. Soc., 2014, 136, 6167 CrossRef CAS
; (j) A. J. Pérez and H. B. Bode, ChemBioChem, 2015, 16, 1588 CrossRef
; (k) K. A. Horner, N. M. Valette and M. E. Webb, Chem. – Eur. J., 2015, 21, 14376 CrossRef CAS
; (l) J. Hoogenboom, H. Zuilhof and T. Wennekes, Org. Lett., 2015, 17, 5550 CrossRef CAS PubMed
; (m) A. Borrmann, O. Fatunsin, J. Dommerholt, A. M. Jonker, D. W. P. M. Löwik, J. C. M. van Hest and F. L. van Delft, Bioconjugate Chem., 2015, 26, 257 CrossRef CAS PubMed
; (n) E. Galardon and D. Padovani, Bioconjugate Chem., 2015, 26, 1013 CrossRef CAS
; (o) E. M. Schneider, M. Zeltner, V. Zlateski, R. N. Grass and W. J. Stark, Chem. Commun., 2016, 52, 938 RSC
; (p) Y. Sun, X. Ma, K. Cheng, B. Wu, J. Duan, H. Chen, L. Bu, R. Zhang, X. Hu, Z. Deng, L. Xing, X. Hong and Z. Cheng, Angew. Chem., Int. Ed., 2015, 54, 5981 CrossRef CAS
; (q) H. Liu, D. Audisio, L. Plougastel, E. Decuypere, D.-A. Buisson, O. Koniev, S. Kolodych, A. Wagner, M. Elhabiri, A. Krzyczmonik, S. Forsback, O. Solin, V. Gouverneur and F. Taran, Angew. Chem., Int. Ed., 2016, 55, 12073 CrossRef CAS
; (r) R. Sen, Di. Gahtory, R. R. Carvalho, B. Albada, F. L. van Delft and H. Zuilhof, Angew. Chem., Int. Ed., 2017, 56, 4130 CrossRef CAS PubMed
; (s) S. Bernard, D. Audisio, M. Riomet, S. Bregant, A. Sallustrau, L. Plougastel, E. Decuypere, S. Gabillet, R. A. Kumar, J. Elyian, M. N. Trinh, O. Koniev, A. Wagner, S. Kolodych and F. Taran, Angew. Chem., Int. Ed., 2017, 56, 15612 CrossRef CAS
; (t) S. Ursuegui, M. Recher, W. Krężel and A. Wagner, Nat. Commun., 2017, 8, 15242 CrossRef CAS PubMed
; (u) X. Li, Z. Liu and S. Dong, RSC Adv., 2017, 7, 44470 RSC
; (v) W. Wang, X. Ji, Z. Du and B. Wang, Chem. Commun., 2017, 53, 1370 RSC
; (w) P. Werther, J. S. Möhler and R. Wombacher, Chem. – Eur. J., 2017, 23, 18216 CrossRef CAS PubMed
; (x) M. Bjerknes, H. Cheng, C. D. McNitt and V. V. Popik, Bioconjugate Chem., 2017, 28, 1560 CrossRef CAS
; (y) L. C.-C. Lee, H. M.-H. Cheung, H.-W. Liu and K. K.-W. Lo, Chem. – Eur. J., 2018, 24, 14064 CrossRef CAS
; (z) A. Herrmann, L. Kaufmann, P. Dey, R. Haag and U. Schedler, ACS Appl. Mater. Interfaces, 2018, 10, 11382 CrossRef CAS
; (a a) C. Favre and F. Friscourt, Org. Lett., 2018, 20, 4213 CrossRef CAS PubMed
; (a b) B. J. Levandowski, R. F. Gamache, J. M. Murphy and K. N. Houk, J. Am. Chem. Soc., 2018, 140, 6426 CrossRef CAS PubMed
; (a c) B. Spangler, S. Yang, C. M. B. Rath, F. Reck and B. Y. Feng, ACS Chem. Biol., 2019, 14, 725 CrossRef CAS
; (a d) P. N. Gunawardene, W. Luo, A. M. Polgar, J. F. Corrigan and M. S. Workentin, Org. Lett., 2019, 21, 5547 CrossRef CAS
; (a e) B. J. Levandowski, N. S. Abularrage, K. N. Houk and R. T. Raines, Org. Lett., 2019, 21, 8492 CrossRef CAS PubMed
; (a f) M. Baalmann, M. J. Ziegler, P. Werther, J. Wilhelm and R. Wombacher, Bioconjugate Chem., 2019, 30, 1405 CrossRef CAS
; (a g) B. J. Levandowski, D. Svatunek, B. Sohr, H. Mikula and K. N. Houk, J. Am. Chem. Soc., 2019, 141, 2224 CrossRef CAS
; (a h) P. N. Gunawardene, J. F. Corrigan and M. S. Workentin, J. Am. Chem. Soc., 2019, 141, 11781 CrossRef CAS
; (a i) Z.-C. Wu and D. L. Boger, J. Am. Chem. Soc., 2019, 141, 16388 CrossRef CAS
; (a j) U. Reisacher, D. Ploschik, F. Rönicke, G. B. Cserép, P. Kele and H.-A. Wagenknecht, Chem. Sci., 2019, 10, 4032 RSC
; (a k) L. Plougastel, M. R. Pattanayak, M. Riomet, S. Bregant, A. Sallustrau, M. Nothisen, A. Wagner, D. Audisio and F. Taran, Chem. Commun., 2019, 55, 4582 RSC
; (a l) X. Zhang, X. Wu, S. Jiang, J. Gao, Z. Yao, J. Deng, L. Zhang and Z. Yu, Chem. Commun., 2019, 55, 7187 RSC
; (a m) V. N. Kozhevnikov, M. E. Deary, T. Mantso, M. I. Panayiotidis and M. T. Sims, Chem. Commun., 2019, 55, 14283 RSC
; (a n) H. Noda, Y. Asada, M. Shibasaki and N. Kumagai, Org. Biomol. Chem., 2019, 17, 1813 RSC
; (a o) R. A. Kumar, M. R. Pattanayak, E. Yen-Pon, J. Eliyan, K. Porte, S. Bernard, M. Riomet, P. Thuéry, D. Audisio and F. Taran, Angew. Chem., Int. Ed., 2019, 58, 14544 CrossRef CAS PubMed
; (a p) M. Riomet, K. Porte, L. Madegard, P. Thuéry, D. Audisio and F. Taran, Org. Lett., 2020, 22, 2403 CrossRef CAS PubMed
; (a q) E. Ros, M. Bellido, X. Verdaguer, L. R. de Pouplana and A. Riera, Bioconjugate Chem., 2020, 31, 933 CrossRef CAS PubMed
; (a r) C. D. Mboyi, D. Vivier, A. Daher, P. Fleurat-Lessard, H. Cattey, C. H. Devillers, C. Bernhard, F. Denat, J. Roger and J.-C. Hierso, Angew. Chem., Int. Ed., 2020, 59, 1149 CrossRef CAS PubMed
.
-
(a) S. Yoshida, A. Shiraishi, K. Kanno, T. Matsushita, K. Johmoto, H. Uekusa and T. Hosoya, Sci. Rep., 2011, 1, 82 CrossRef PubMed
; (b) S. Yoshida, T. Nonaka, T. Morita and T. Hosoya, Org. Biomol. Chem., 2014, 12, 7489 RSC
; (c) T. Meguro, S. Yoshida and T. Hosoya, Chem. Lett., 2017, 46, 1137 CrossRef CAS
; (d) S. Yoshida, K. Kanno, I. Kii, Y. Misawa, M. Hagiwara and T. Hosoya, Chem. Commun., 2018, 54, 3705 RSC
; (e) T. Meguro, N. Terashima, H. Ito, Y. Koike, I. Kii, S. Yoshida and T. Hosoya, Chem. Commun., 2018, 54, 7904 RSC
; (f) S. Yoshida, J. Tanaka, Y. Nishiyama, Y. Hazama, T. Matsushita and T. Hosoya, Chem. Commun., 2018, 54, 13499 RSC
; (g) T. Meguro, S. Yoshida, K. Igawa, K. Tomooka and T. Hosoya, Org. Lett., 2018, 20, 4126 CrossRef CAS PubMed
; (h) T. Meguro, S. Chen, K. Kanemoto, S. Yoshida and T. Hosoya, Chem. Lett., 2019, 48, 582 CrossRef CAS
; (i) S. Yoshida, S. Goto, Y. Nishiyama, Y. Hazama, M. Kondo, T. Matsushita and T. Hosoya, Chem. Lett., 2019, 48, 1038 CrossRef CAS
; (j) T. Meguro, Y. Sakata, T. Morita, T. Hosoya and S. Yoshida, Chem. Commun., 2020, 56, 4720 RSC
.
-
(a) S. Yoshida, Y. Hatakeyama, K. Johmoto, H. Uekusa and T. Hosoya, J. Am. Chem. Soc., 2014, 136, 13590 CrossRef CAS PubMed
; (b) S. Yoshida, T. Kuribara, H. Ito, T. Meguro, Y. Nishiyama, F. Karaki, Y. Hatakeyama, Y. Koike, I. Kii and T. Hosoya, Chem. Commun., 2019, 55, 3556 RSC
.
- For an alternative approach, see; R. R. Ramsubhag and G. B. Dudley, Org. Biomol. Chem., 2016, 14, 5028 RSC
.
-
(a) G. Wittig and H.-L. Dorsch, Liebigs Ann. Chem., 1968, 711, 46 CrossRef CAS
; (b) G. Wittig and S. Fischer, Chem. Ber., 1972, 105, 3542 CrossRef CAS
; (c) G. Gröger, U. Behrens and F. Olbrich, Organometallics, 2000, 19, 3354 CrossRef
; (d) M. Shelbourne, X. Chen, T. Brown and A. H. El-Sagheer, Chem. Commun., 2011, 47, 6257 RSC
; (e) A. Das, C. Dash, M. Yousufuddin, M. A. Celik, G. Frenking and H. V. R. Dias, Angew. Chem., Int. Ed., 2012, 51, 3940 CrossRef CAS PubMed
; (f) A. Das, C. Dash, M. A. Celik, M. Yousufuddin, G. Frenking and H. V. R. Dias, Organometallics, 2013, 32, 3135 CrossRef CAS
; (g) P. Gobbo, T. Romagnoli, S. M. Barbon, J. T. Price, J. Keir, J. B. Gilroy and M. S. Workentin, Chem. Commun., 2015, 51, 6647 RSC
.
- M. A. Bennett and H. P. Schwemlein, Angew. Chem., Int. Ed. Engl., 1989, 28, 1296 CrossRef
.
-
(a) X. Ning, J. Guo, M. A. Wolfert and G.-J. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253 CrossRef CAS PubMed
; (b) X. Ning, R. P. Temming, J. Dommerholt, J. Guo, D. B. Ania, M. F. Debets, M. A. Wolfert, G.-J. Boons and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 3065 CrossRef CAS PubMed
.
-
(a) R. Ni, N. Mitsuda, T. Kashiwagi, K. Igawa and K. Tomooka, Angew. Chem., Int. Ed., 2015, 54, 1190 CrossRef CAS PubMed
; (b) Y. Kawasaki, Y. Yamanaka, Y. Seto, K. Igawa and K. Tomooka, Chem. Lett., 2019, 49, 495 CrossRef
.
- Complexes of 1a with transition metals formed as precipitates.
- Although further detailed studies are required, the selectivity would be concerned with the following two factors; (1) the coordination strength of silver or gold with cycloalkynes is weaker than that of copper and (2) electron-rich and highly strained natures of BCNs strengthen the coordination.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization for the new compounds including NMR spectra. See DOI: 10.1039/d0cc04606j |
| This journal is © The Royal Society of Chemistry 2020 |