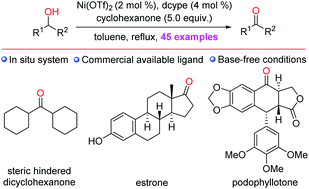
Abstract
An efficient nickel(II)-catalyzed transfer dehydrogenation oxidation of alcohols is reported that relies on cyclohexanone as the formal oxidant and does not require the use of an external base. The synthetic utility of this protocol is demonstrated via the facile oxidation of structurally complicated natural products.


 Please wait while we load your content...
Please wait while we load your content...