Stereoselective aminosulfonylation of alkynes: an approach to access (Z)-β-amino vinylsulfones†
Abstract
The stereoselective sulfur–nitrogen difunctionalization (aminosulfonylation) across the alkyne framework in a syn mode is accomplished using sodium sulfinates and azoles, in the presence of I2/base. This strategy is applicable to a variety of terminal alkynes, sodium sulfinates, and azoles as reaction partners, affording (Z)-β-amino vinylsulfones in good yields.
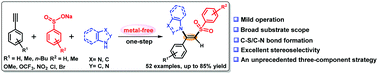


 Please wait while we load your content...
Please wait while we load your content...