Photoredox-catalyzed sulfonylation of difluoroenoxysilanes with the insertion of sulfur dioxide†
Abstract
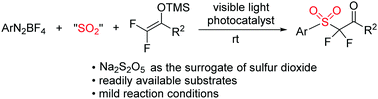
A photoredox-catalyzed three-component reaction of aryldiazonium tetrafluoroborates with sodium metabisulfite and 2,2-difluoro enol silyl ethers is described. By using sodium metabisulfite as the source of sulfur dioxide, this method provides an elegant access to α,α-difluoro-β-ketosulfones in moderate to good yields under mild conditions, and features a broad substrate scope and wide functional group tolerance. Both of the difluoromethyl group and sulfone moiety can be introduced in a single step. Based on the experimental results, a single-electron transfer pathway is proposed with the insertion of sulfur dioxide.


 Please wait while we load your content...
Please wait while we load your content...