Open Access Article
Open Access ArticleTerminal-oxidant-free photocatalytic C–H alkylations of heteroarenes with alkylsilicates as alkyl radical precursors†
Gun
Ikarashi
ab,
Tatsuya
Morofuji
 *a and
Naokazu
Kano
*a and
Naokazu
Kano
 *a
*a
aDepartment of Chemistry, Faculty of Science, Gakushuin University 1-5-1 Mejiro, Toshima-ku, Tokyo 171-8588, Japan. E-mail: tatsuya.morofuji@gakushuin.ac.jp; naokazu.kano@gakushuin.ac.jp
bDepartment of Chemistry, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
First published on 22nd July 2020
Abstract
We report the photocatalytic C–H alkylations of heteroarenes with alkylsilicates bearing C,O-bidentate ligands under acidic conditions. Irradiation of heteroaromatics in the presence of the silicates and trifluoroacetic acid produced the corresponding alkylated compounds. The present reaction system does not require any terminal oxidant although the reaction seems to be a formal oxidation reaction. This study demonstrates that alkylsilicates can be used in photocatalytic radical chemistry under acidic conditions.
Heteroarenes are important components of natural products and active pharmaceutical ingredients.1 The radical substitution of a C–H bond of a protonated heteroarene, the Minisci reaction, is a well-known versatile method for derivatizing heteroarenes,2 and many Minisci-type reactions have been developed over the past several decades. Carboxylic acids,3 alkyl halides,4 activated esters,5 peroxides,6 alcohols,7 boronic acids,8 sulfinate salts,9 alkenes,10 alkyltrifluoroborates,11 and 1,4-dihydropyridines12 have all been used as radical precursors in these reactions. In addition, although most Minisci-type reactions require stoichiometric amounts of an oxidant, oxidant-free versions, which are expected to serve as mild and clean methods for alkylating heteroarenes, have been developed recently.3f,11d Despite great effort, there are only a few examples of Minisci-type reactions that use organosilicon compounds.13 Only benzylsilanes and silicon compounds bearing single chalcogen atoms at their α-positions have been used as radical precursors in Minisci-type reactions (Fig. 1a) because most silicon compounds, whose carbon–silicon bonding orbitals do not interact with π electrons or lone pairs, have very high oxidation potentials, making it difficult to generate alkyl radicals through oxidation.14 In this decade, pentacoordinated alkylsilicates 1 bearing pairs of catecholate ligands have turned out to be alkyl radical precursors with low oxidation potentials.15 Because of lower oxidation potentials, alkysilicates 1 have been used in place of alkyltrifluoroborates that produce toxic boron fluoride byproducts and are poorly soluble in most organic solvents. As one example, desulfonative alkylation of N-heteroaryl sulfones using bis(catecholato)silicates under non-acidic conditions was reported.15d However, such alkylsilicates, which are unstable to acid, have not been used in reactions under acidic conditions, such as Minisci-type C–H alkylations of heteroarenes.
 | ||
| Fig. 1 (a) Organosilicon compounds as radical precursors. (b) Synthesis of alkylsilicates 2. (c) This work: C–H alkylation of heteroarenes with alkylsilicates as alkyl radical precursors. | ||
With this background in mind, we focused our attention on alkylsilicates 2 bearing pairs of [-C6H4-2-C(CF3)2O-] C,O-bidentate ligands.16,17 Alkylsilicates 2 can be directly synthesized by the reaction of an alkyllithium or alkylmagnesium bromide with Martin's spirosilane 3 (Fig. 1b).16a,b Beneficially, alkylsilicates 2 are stable to water and dissolve well in common organic solvents. Moreover, 2 remain intact under acidic conditions.16c Hence, we envisaged that 2 would provide opportunities for the development of new photoredox reactions using organosilicon reagents as radical precursors in acidic media. Herein, we report the photocatalytic C–H alkylation of heteroarenes using alkylsilicates 2 (Fig. 1c). Notably, the present reaction system does not require any terminal oxidant although the reaction seems to be a formal oxidation reaction.
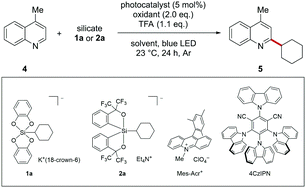
To optimize the reaction conditions, 4-methylquinoline (4) and cyclohexylsilicate 2a were selected as the model substrate and radical precursor, respectively (Table 1). We first carried out the reaction referring to the reaction conditions of photocatalytic Minisci-type reactions using alkyltrifluoroborates as radical precursors.11c Irradiation of 4, 2a, (NH4)2S2O8, and 9-mesityl-10-methylacridinium perchlorate (Mes-Acr+) as the photocatalyst in CH3CN/H2O gave the desired C–H alkylated product 5 in 81% yield (entry 1). Photocatalyst was essential for achieving the alkylation (entries 2 and 3). Surprisingly, the reaction proceeded well in the absence of (NH4)2S2O8 (entry 4), in contrast to the Minisci-type reactions with alkyltrifluoroborates. We considered the possibility that a trace amount of oxygen present in the solution acted as the oxidant, but the desired product was hardly obtained in an oxygen atmosphere (entry 5). Irradiation with blue light, the presence of an acid, and the photocatalyst were all essential for the reaction to progress (entries 6–8). Using CH2Cl2 as the solvent improved the yield of 5 (entry 9). As expected, cyclohexylsilicate 1a, which bears two catecholate ligands, failed to give the desired product (entry 10). The reaction with 4CzIPN as the photocatalyst proceeded, albeit with a slightly lower yield of 5 (entry 11).
| Entry | Silicate | Photocatalyst | Oxidant | Solvent | Yielda [%] |
|---|---|---|---|---|---|
| 4-Methylquinoline (4, 0.1 mmol), cyclohexylsilicate 1a or 2a (0.12 mmol), oxidant (0.2 mmol), photocatalyst (5 mol%), and TFA (0.11 mmol) were stirred at 23 °C in solvent (4 mL) for 24 h and irradiated with blue light.a Determined by 1H NMR spectroscopy.b The reaction was carried out in the dark.c TFA was not added. | |||||
| 1 | 2a | Mes-Acr+ | (NH4)2S2O8 | CH3CN/H2O | 81 |
| 2 | 2a | None | (NH4)2S2O8 | CH3CN/H2O | 0 |
| 3b | 2a | None | (NH4)2S2O8 | CH3CN/H2O | 0 |
| 4 | 2a | Mes-Acr+ | None | CH3CN/H2O | 71 |
| 5 | 2a | Mes-Acr+ | O2 (1 atm) | CH3CN/H2O | Trace |
| 6b | 2a | Mes-Acr+ | None | CH3CN/H2O | 0 |
| 7c | 2a | Mes-Acr+ | None | CH3CN/H2O | Trace |
| 8 | 2a | None | None | CH3CN/H2O | Trace |
| 9 | 2a | Mes-Acr+ | None | CH2Cl2 | 87 |
| 10 | 1a | Mes-Acr+ | None | CH2Cl2 | 0 |
| 11 | 2a | 4CzIPN | None | CH2Cl2 | 73 |
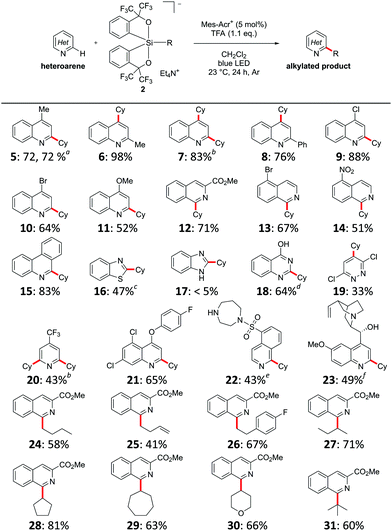
With the optimal conditions in hand, the generality of this Minisci-type C–H alkylation reaction was evaluated. As shown in Table 2, a diverse range of heteroarenes was alkylated under the optimized conditions. The reaction also efficiently produced 5 on the gram scale. 2-Methylquinoline also gave the desired product 6 in high yield, while unsubstituted quinoline gave the 2,4-dialkylated product 7 in 83% yield using 2.2 equivalents of cyclohexylsilicate 2a. Quinolines with a variety of substituents, including phenyl, chloro, bromo, and methoxy, also gave the corresponding products 8–11. Isoquinolines, as well as unsubstituted phenanthridine and benzothiazole, were also amenable to the protocol to produce 12–16, respectively. As expected, the reaction with benzimidazole resulted in almost no conversion to 17 because of the electron-rich nature of its C2 site,11c while quinazolinone and 3,6-dichloropyridazine afforded the desired products 18 and 19. 4-(Trifluoromethyl)pyridine was converted into the 2,6-dialkylated product 20. Furthermore, this Minisci-type alkylation protocol was also successfully used to functionalize complex bioactive molecules. For instance, the fungicide quinoxyfen was alkylated at the C2 position of its quinoline ring to give 21 in 65% yield. Fasudil, a potent vasodilator bearing a free NH group, was selectively alkylated at the C1 position to give 22 in 43% yield, which contrasts sharply with the previous photocatalytic Minisci-type alkylation using persulfate as the terminal oxidant that required protection of the NH group.3c Quinine gave the corresponding product 23 in 49% yield.
| Heteroarene (0.2 mmol), alkylsilicate 2 (0.24 mmol), Mes-AcrClO4 (5 mol%), and TFA (0.22 mmol) were stirred at 23 °C in CH2Cl2 (4 mL) for 24 h while irradiated with blue light. Yields are isolated yields.a The reaction was performed on a gram scale.b 2.2 equiv. of 2a was used.c Performed in a CH2Cl2/H2O mixture (1/1).d Performed in a CH3CN/H2O mixture (1/1).e 2.1 equiv. of TFA was used and the product was isolated in N-Boc protected form.f 2.1 equiv. of TFA was used and 1,1,1,3,3,3-hexafluoro-2-propanol was used as the solvent. |
|---|
|
The scope of the silicate was subsequently investigated. Primary alkylsilicates could be used in this reaction to give 24–26, although primary alkyl radicals are less stable than secondary and tertiary alkyl radicals. Both acyclic and cyclic secondary alkylsilicates as well as tertiary alkylsilicates were applicable to the present reaction system to give 27–31.
The following experiments were performed to obtain mechanistic insight into the present reaction. Stern–Volmer quenching experiments revealed that 2a efficiently quenches the excited state of the photocatalyst, whereas the heteroarene, TFA, the protonated heteroarene, and spirosilane 3 did not (see ESI†). These results suggest that oxidation of 2a is the key initiation step for the C–H alkylation reaction. We next performed a radical clock experiment. Alkylation of 4 with 5-hexenylsilicate 2b under the standard conditions gave the ring-closed product 32 without the formation of the linear product 32′ (Scheme 1a), confirming the radical pathway.
 | ||
| Scheme 1 Mechanistic studies. (a) A radical clock experiment. (b) Observing the intermediate. (c) The dehydrogenation reaction. (d) Proposed reaction mechanism. | ||
The present method does not require any terminal oxidant, whereas previous oxidant-free Minisci-type reactions include electrochemical oxidation3f,11d or hydrogen evolution.3d,3e To gain some insight into the alternative oxidative process operating in the present system, we examined the crude products from the reactions of alkylsilicates 2a with benzothiazole as a substrate. 1H NMR spectroscopy revealed the formation of 2-cyclohexyl-2,3-dihydrobenzo[d]thiazole (16′) in addition to the desired 2-cyclohexylbenzothiazole (16) (Scheme 1b), which suggests that the hydrogenated form of the desired product is a reaction intermediate. To address this possibility, we investigated reaction conditions for the conversion of 16′ into 16 (Scheme 1c). The addition of alkylsilicate 2a, spirosilane 3, TFA, or Mes-Acr+ to a CD2Cl2 solution of 16′ resulted in no reaction. However, the dehydrogenated product 16 was observed in 38% yield by 1H NMR spectroscopy after irradiation with blue light in the presence of Mes-Acr+. Furthermore, irradiation of 16′ in the presence of both the photocatalyst and TFA produced 16 in quantitative yield, strongly suggesting the intermediacy of 16′ during the formation of 16.
We propose a possible mechanism for the photocatalytic C–H alkylation reaction (Scheme 1d). First, Mes-Acr+ is excited by visible light and oxidizes alkylsilicate 2, releasing alkyl radical R˙ and spirosilane 3. This process is energetically feasible because the reduction potential of Mes-Acr+* (Ered = +2.06 V vs. SCE)18 is more positive than the oxidation potential of cyclohexylsilicate 2a (Ered = +1.47 V vs. SCE). The generated alkyl radical R˙ reacts with the protonated heteroarene to form radical cation I. Single electron transfer (SET) from Mes-Acr˙ to radical cation I then forms intermediate II. Quenching experiments indicate that the oxidation of intermediate II by the excited state of the photocatalyst would generate I (see ESI†). Reaction of I and II generates radical III, hydrogen molecule, and protonated desired product, which is deprotonated to give the final product. Protonation of III gives I again.
In summary, we developed a new protocol for the photocatalytic Minisci-type C–H alkylations of heteroarenes using alkylsilicates as alkyl radical precursors. This method does not require any terminal oxidant, which makes it possible to functionalize various heteroarenes in a mild and clean reaction system. A variety of primary, secondary, and tertiary alkyl groups was directly incorporated into various electron-deficient heteroarenes in an efficient manner. Mechanistic studies suggest that this terminal-oxidant-free alkylation involves the photocatalytic formation of the hydrogenated form of the desired product followed by photocatalytic dehydrogenation. This study clearly demonstrates that alkylsilicates can be used in photocatalytic radical chemistry under acidic conditions.
This research was partially supported by a MEXT-supported program for Strategic Research Foundations at Private Universities, JSPS KAKENHI Grant Number JP19K15570, the Tokyo Ohka Foundation for The Promotion of Science and Technology, Merck & Co., Inc and the Fukuoka Naohiko Foundation. This research was also supported by crowdfunding (academist). The authors thank Yumi Hayashi, Subaru Kawasaki, Yuki Miyabayashi, Kazufumi Nakano, Nobu, Kenta Tanaka, Yoshiyuki Yagi, and Jun Yamaoka for funding. The authors thank Prof. Koichi Iwata and Dr. Akira Takakado for their assistance with the Stern–Volmer quenching experiments. The authors thank Yu Matsui for her assistance with measuring melting points and oxidation potentials. DFT calculations were performed using Research Center for Computational Science, Okazaki, Japan.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. F. Pozharskii, A. T. Soldatenkov and A. R. Katritzky, Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, Wiley, Hoboken, 2nd edn, 2011 Search PubMed.
- (a) F. Minisci, E. Vismara and F. Fontana, Heterocycles, 1989, 28, 489–519 CrossRef CAS; (b) M. A. J. Duncton, Med. Chem. Commun., 2011, 2, 1135–1161 RSC; (c) R. S. J. Proctor and R. J. Phipps, Angew. Chem. Int. Ed., 2019, 58, 13666–13699 CrossRef CAS PubMed.
- (a) F. Minisci, R. Mondelli, G. P. Gardini and O. Porta, Tetrahedron, 1972, 28, 2403–2413 CrossRef CAS; (b) N. Kaur, X. Lu, M. C. Gershengorn and R. Jain, J. Med. Chem., 2005, 48, 6162–6165 CrossRef CAS PubMed; (c) R. A. Garza-Sanchez, A. Tlahuext-Aca, G. Tavakoli and F. Glorius, ACS Catal., 2017, 7, 4057–4061 CrossRef CAS; (d) J. Lin, Z. Li, J. Kan, S. Huang, W. Su and Y. Li, Nat. Commun., 2017, 8, 14353 CrossRef PubMed; (e) W.-F. Tian, C.-H. Hu, K.-H. He, X.-Y. He and Y. Li, Org. Lett., 2019, 21, 6930–6935 CrossRef CAS PubMed; (f) X.-L. Lai, X.-M. Shu, J. Song and H.-C. Xu, Angew. Chem., Int. Ed., 2020, 59, 10626–10632 CrossRef CAS PubMed.
- (a) F. Minisci, E. Vismara and F. Fontana, J. Org. Chem., 1989, 54, 5224–5227 CrossRef CAS; (b) M. A. J. Duncton, M. A. Estiarte, R. J. Johnson, M. Cox, D. J. R. O’Mahony, W. T. Edwards and M. G. Kelly, J. Org. Chem., 2009, 74, 6354–6357 CrossRef CAS PubMed.
- (a) W.-M. Cheng, R. Shang, M.-C. Fu and Y. Fu, Chem. – Eur. J., 2017, 23, 2537–2541 CrossRef CAS PubMed; (b) W.-M. Cheng, R. Shang and Y. Fu, ACS Catal., 2017, 7, 907–911 CrossRef CAS; (c) M.-C. Fu, R. Shang, B. Zhao, B. Wang and Y. Fu, Science, 2019, 363, 1429–1434 CrossRef CAS PubMed.
- D. A. Dirocco, K. Dykstra, S. Krska, P. Vachal, D. V. Conway and M. Tudge, Angew. Chem., Int. Ed., 2014, 53, 4802–4806 CrossRef CAS PubMed.
- (a) J. Jin and D. W. C. Macmillan, Nature, 2015, 525, 87–90 CrossRef CAS PubMed; (b) S. P. Pitre, M. Muuronen, D. A. Fishman and L. E. Overman, ACS Catal., 2019, 9, 3413–3418 CrossRef CAS.
- I. B. Seiple, S. Su, R. A. Rodriguez, R. Gianatassio, Y. Fujiwara, A. L. Sobel and P. S. Baran, J. Am. Chem. Soc., 2010, 132, 13194–13196 CrossRef CAS PubMed.
- (a) Y. Fujiwara, J. A. Dixon, F. O’Hara, E. D. Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herlé, N. Sach, M. R. Collins, Y. Ishihara and P. S. Baran, Nature, 2012, 492, 95–99 CrossRef CAS PubMed; (b) F. O’Hara, D. G. Blackmond and P. S. Baran, J. Am. Chem. Soc., 2013, 135, 12122–12134 CrossRef PubMed; (c) R. Gianatassio, S. Kawamura, C. L. Eprile, K. Foo, J. Ge, A. C. Burns, M. R. Collins and P. S. Baran, Angew. Chem., Int. Ed., 2014, 53, 9851–9855 CrossRef CAS PubMed.
- (a) X. Ma and S. B. Herzon, J. Am. Chem. Soc., 2016, 138, 8718–8721 CrossRef CAS PubMed; (b) J. C. Lo, D. Kim, C.-M. Pan, J. T. Edwards, Y. Yabe, J. Gui, T. Qin, S. Gutiérrez, J. Giacoboni, M. W. Smith, P. L. Holland and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 2484–2503 CrossRef CAS PubMed.
- (a) G. A. Molander, V. Colombel and V. A. Braz, Org. Lett., 2011, 13, 1852–1855 CrossRef CAS PubMed; (b) J. K. Matsui and G. A. Molander, Org. Lett., 2017, 19, 950–953 CrossRef CAS PubMed; (c) J. K. Matsui, D. N. Primer and G. A. Molander, Chem. Sci., 2017, 8, 3512–3522 RSC; (d) H. Yan, Z.-W. Hou and H.-C. Xu, Angew. Chem., Int. Ed., 2019, 58, 4592–4595 CrossRef CAS PubMed.
- Á. Gutiérrez-Bonet, C. Remeur, J. K. Matsui and G. A. Molander, J. Am. Chem. Soc., 2017, 139, 12251–12258 CrossRef PubMed.
- J. Dong, X. Wang, Z. Wang, H. Song, Y. Liu and Q. Wang, Org. Chem. Front., 2019, 6, 2902–2906 RSC.
- (a) J. Yoshida, T. Maekawa, T. Murata, S. Matsunaga and S. Isoe, J. Am. Chem. Soc., 1990, 112, 1962–1970 CrossRef CAS; (b) J. Biedermann, H. M. R. Wilkening, F. Uhlig and I. Hanzu, Electrochem. Commun., 2019, 102, 13–18 CrossRef CAS.
- (a) V. Corcé, L.-M. Chamoreau, E. Derat, J.-P. Goddard, C. Ollivier and L. Fensterbank, Angew. Chem., Int. Ed., 2015, 54, 11414–11418 CrossRef PubMed; (b) M. Jouffroy, D. N. Primer and G. A. Molander, J. Am. Chem. Soc., 2016, 138, 475–478 CrossRef CAS PubMed; (c) C. Lévêque, L. Chenneberg, V. Corcé, C. Ollivier and L. Fensterbank, Chem. Commun., 2016, 52, 9877–9880 RSC; (d) Z.-J. Wang, S. Zheng, J. K. Matsui, Z. Lu and G. A. Molander, Chem. Sci., 2019, 10, 4389–4393 RSC.
- (a) E. F. Perozzi and J. C. Martin, J. Am. Chem. Soc., 1979, 101, 1591–1593 CrossRef CAS; (b) W. H. Stevenson, S. Wilson, J. C. Martin and W. B. Farnham, J. Am. Chem. Soc., 1985, 107, 6340–6352 CrossRef CAS; (c) K. C. K. Swamy, V. Chandrasekhar, J. J. Harland, J. M. Holmes, R. O. Day and R. R. Holmes, J. Am. Chem. Soc., 1990, 112, 2341–2348 CrossRef CAS.
- For application of the spirosilane bearing a pair of the C,O-bidentate ligands [–C6H4–2-C(CF3)2O–]: (a) M. Kira, K. Sato and H. Sakurai, J. Am. Chem. Soc., 1988, 110, 4599–4602 CrossRef CAS; (b) D. A. Dixon, W. R. Hertler, D. B. Chase, W. B. Farnham and F. Davidson, Inorg. Chem., 1988, 27, 4012–4018 CrossRef CAS; (c) S. K. Chopra and J. C. Martin, J. Am. Chem. Soc., 1990, 112, 5342–5343 CrossRef CAS; (d) H. Lenormand, J.-P. Goddard and L. Fensterbank, Org. Lett., 2013, 15, 748–751 CrossRef CAS PubMed; (e) F. Medici, G. Gontard, E. Derat, G. Lemière and L. Fensterbank, Organometallics, 2018, 37, 517–520 CrossRef CAS; (f) F. Medici, J. Maury, G. Lemiere and L. Fensterbank, Chem. – Eur. J., 2019, 25, 9438–9442 CrossRef CAS PubMed; (g) K. Sakai, K. Oisaki and M. Kanai, Adv. Synth. Catal., 2020, 362, 337–343 CrossRef CAS.
- K. Ohkubo, K. Mizushima, R. Iwata and S. Fukuzumi, Chem. Sci., 2011, 2, 715–722 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, DFT calculations and NMR data. See DOI: 10.1039/d0cc03286g |
| This journal is © The Royal Society of Chemistry 2020 |