Asymmetric catalysis with a chiral-at-osmium complex†
Abstract
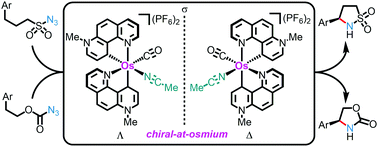
The first example of a chiral osmium catalyst is reported in which the overall chirality originates exclusively from a stereogenic metal center (metal-centered chirality) with all coordinating ligands being achiral. The non-C2-symmetric chiral-at-metal complex contains two cyclometalated 7-methyl-1,7-phenanthrolinium heterocycles which can be described as two chelating pyridylidene remote N-heterocyclic carbene (rNHC) ligands. The octahedral coordination sphere is completed with one CO and one acetonitrile ligand. A monodentate chiral oxazoline ligand is used as a chiral auxiliary ligand to obtain enantiomerically pure chiral-at-osmium complexes (>99 : 1 e.r.). Finally, it is demonstrated that the developed chiral-at-osmium complex is suitable for ring-closing enantioselective C(sp3)–H aminations, including the first example of catalytic enantioselective cyclizations of azidoformates to chiral 2-oxazolidinones.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...