Open Access Article
Open Access ArticleFormaldehyde tert-butyl hydrazone as a formyl anion equivalent: asymmetric addition to carbonyl compounds†
Esteban
Matador‡
 a,
María
de Gracia Retamosa‡
a,
María
de Gracia Retamosa‡
 a,
David
Monge
a,
David
Monge
 *a,
Rosario
Fernández
*a,
Rosario
Fernández
 *a and
José M.
Lassaletta
*a and
José M.
Lassaletta
 *b
*b
aDepartamento de Química Orgánica, Universidad de Sevilla and Centro de Innovación Avanzada (ORFEO-CINQA), C/Prof. García González, 1, 41012 Sevilla, Spain. E-mail: dmonge@us.es; ffernan@us.es
bInstituto de Investigaciones Químicas (CSIC-US) and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Avda. Américo Vespucio, 49, 41092 Sevilla, Spain. E-mail: jmlassa@iiq.csic.es
First published on 23rd June 2020
Abstract
The asymmetric 1,2-addition of formyl anion equivalents to carbonyl compounds is a powerful synthetic tool that ideally provide access to highly functionalizable α-hydroxy aldehydes in an enantioselective fashion. In this context, the nucleophilic character of formaldehyde hydrazones, together with their remarkable stability as monomeric species, has been exploited for the functionalization of diverse carbonyl compounds, using initially auxiliary-based methodologies and, more recently, catalytic enantioselective versions. This feature article highlights our research progress employing formaldehyde tert-butyl hydrazone as a versatile formyl anion equivalent, in combination with bifunctional H-bonding organocatalysis. The design and optimization of different catalytic systems, focusing on a dual activation of both reagents, is reviewed, as well as the racemization free unmasking of the formyl group and representative product transformations for the construction of valuable, densely functionalyzed chiral building blocks.
1. Introduction
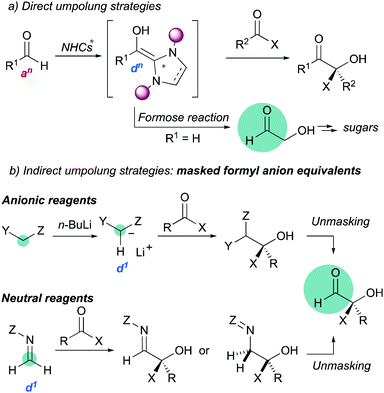
Umpolung strategies for inverting the natural reactivity of functional groups are powerful synthetic tools for generation of molecular complexity in organic synthesis.1 In particular, the asymmetric 1,2-addition of acyl anion equivalents (d1 reagents) to carbonyl compounds provides direct access to valuable functionalized alcohols.2 Most direct nucleophilic acylations are catalyzed by N-heterocyclic carbenes (Scheme 1a),3 but this strategy fails in the case of formaldehyde due to oligomerizations (the formose reaction).4 Therefore, several masked formyl anion reagents (anionic or neutral) have been developed to circumvent such limitation (Scheme 1b).5 These strategies are based on two key steps: (i) asymmetric C–C bond formation, and (ii) subsequent unmasking to reveal the formyl group.Approaches based on anionic reagents require in general harsh, strongly basic conditions and catalytic asymmetric variants have not been developed. Whereas this feature article will focus on the use of the more versatile neutral formyl anion equivalents in asymmetric catalysis, a selection of chiral auxiliary-based reagents is also shown, with the aim to provide a clear background of the topic. It should be noted that, for some reagents, the catalytic systems are highly reminiscent of the corresponding stoichiometric methods that inspired them and for others, the catalytic enantioselective reactions remain elusive. Key aspects which have allowed us to develop efficient reactions employing formaldehyde tert-butyl hydrazone (FTBH) as masked d1 reagent in combination with bifunctional organocatalysts are highlighted.
2. Background: from chiral auxiliary-based reagents to enantioselective reactions
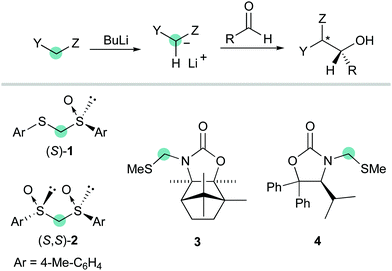
2.1. Anion-stabilized reagents
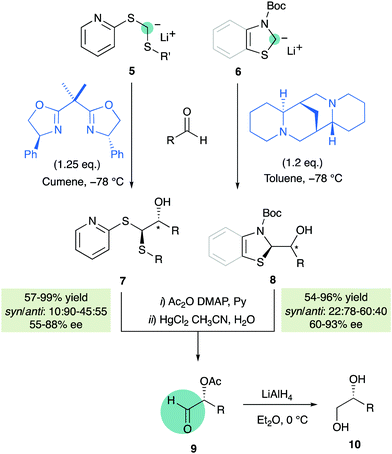
A number of studies have been focused on diastereoselective additions to aldehydes accomplished by using sulfur-stabilized carbanions carrying various chiral auxiliaries such as dithioacetal mono-S-oxide (S)-1,6C2-symmetric bis-sulfoxide (S,S)-2,7 as well as camphor- or valine-derived oxazolidinone S,N-acetals 38 and 49 (Scheme 2).Exploiting again the carbanion stabilizing properties of sulfur, Toru and co-workers reported in 2004 some non-catalytic enantioselective approaches employing achiral α-lithiated dithioacetals 510 or N-Boc-thiazoline/benzothiazoline 611 in the presence of stoichiometric amounts of enantiopure additives such as bis(oxazolines) or (−)sparteine, respectively (Scheme 3). Acetylation of the primary adducts followed by treatment with mercury(II) chloride afforded aldehydes 9 which were directly transformed into enantiomerically enriched diols 10.
As mentioned before, however, the development of catalytic enantioselective versions of the above-mentioned methodologies have been hampered by the strongly basic character of such anionic reagents, incompatible with many catalysts and functional groups, that underwent undesired side reactions. Moreover, unmasking of the formyl group from the primary adducts is still a major difficulty, requiring toxic mercury salts, iodine reagents or multistep sequences.
2.2. Neutral reagents: N,N-dialkyl hydrazones
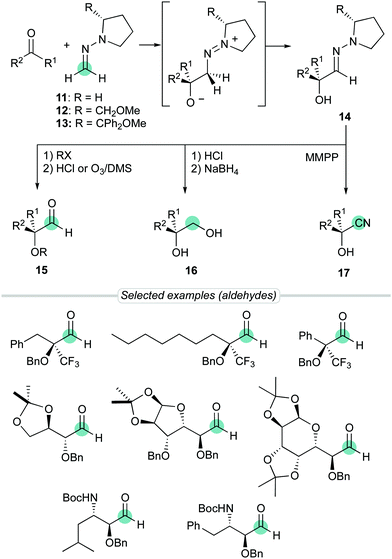
Over the years, we have exploited the enhanced aza-enamine (nucleophilic) character of formaldehyde N,N-dialkyl hydrazones for the stereoselective introduction of single-carbon functional groups into more complex molecular scaffolds.12 In particular, the enhanced reactivity provided by the pyrrolidine ring was exploited to perform diastereoselective additions to diverse electrophiles, including carbonyl compounds. Thus, the simplest achiral derivative 11, along with chiral proline derivatives such as SAMP [(S)-1-amino-2(methoxymethyl)pyrrolidine]hydrazone 1213 or analogue 13 have been successfully employed in reactions with chiral α-alkoxy- and α-amino aldehydes14 (substrate-controlled stereoselectivity) and simple aldehydes15 or trifluoromethyl ketones16 (reagent-controlled stereoselectivity) to afford densely functionalized α-hydroxy hydrazones 14 (Scheme 4). These procedures, based in a soft and neutral reagent, not only benefit of milder reaction conditions, but also provides a remarkable versatility associated to efficient transformations of the hydrazone moiety. The primary targets, aldehydes 15, can be readily synthesized by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond cleavage using acid hydrolysis or ozonolysis,17 and eventually reduced to diols 16. Alternatively, cyanohydrin derivatives 17 can also be easily obtained after oxidative N–N bond cleavage (aza-Cope type elimination) promoted by magnesium monoperoxyphthalate hexahydrate (MMPP).18
N bond cleavage using acid hydrolysis or ozonolysis,17 and eventually reduced to diols 16. Alternatively, cyanohydrin derivatives 17 can also be easily obtained after oxidative N–N bond cleavage (aza-Cope type elimination) promoted by magnesium monoperoxyphthalate hexahydrate (MMPP).18
 | ||
| Scheme 4 Chiral auxiliary-based formaldehyde N,N-dialkyl hydrazones as neutral masked formyl anion equivalents. | ||
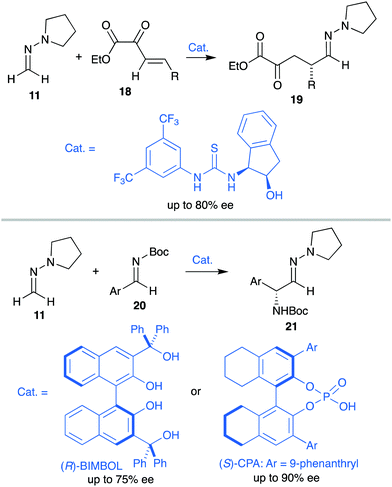
The development of catalytic enantioselective versions of these reactions proved to be a difficult task due to the sensitivity of hydrazones toward most Lewis acidic metal complexes, usually employed for the activation of weakly electrophilic substrates.19 The milder nature of organocatalytic activation strategies, however, appeared to solve these compatibility issues.20 Thus, H-bonding activation by thioureas was identified as a suitable strategy for the conjugate addition of achiral pyrrolidine derivative 11 to β,γ-unsaturated α-ketoesters 18,21 while LUMO-lowering activation by axially chiral BINOL, phosphoric (CPA) or dicarboxylic acid derivatives allowed 1,2-addition of 11 to N-Boc protected imines 20 (Scheme 5).22 The corresponding products 19 and 21, respectively, were obtained with moderate average enantioselectivities in both cases, underlining the need of more efficient activation modes. Moreover, none of these strategies provided satisfactory results in 1,2-additions of N,N-dialkyl hydrazones to carbonyl compounds. These limitations prompted us to explore a dual activation mode as an alternative strategy for the development of the targeted enantioselective formylation of these types of substrates.
 | ||
| Scheme 5 Seminal enantioselective organocatalytic reactions employing formaldehyde N,N-dialkyl hydrazones. | ||
3. Asymmetric organocatalytic 1,2-additions of formaldehyde tert-butyl hydrazone to carbonyl compounds
3.1. Hypothesis and design of the catalytic system
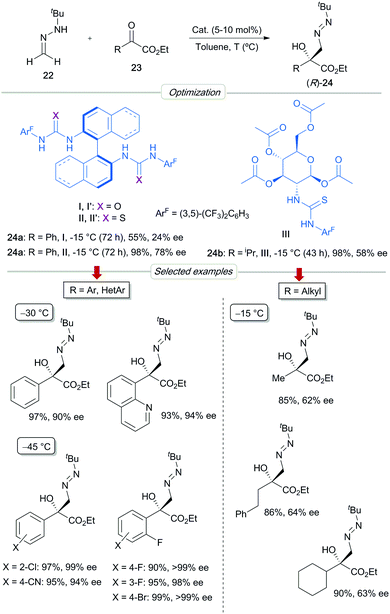
Due to the N,N-disubstitution in hydrazones such as 11, the attack of the nitrogen atom to neutral electrophilic reagents results in the formation of zwitterionic compounds in a non-productive, reversible process. In monosubstituted analogues, however, the attack of the more nucleophilic nitrogen atom must be avoided to prevent undesired side reactions. In this context, Baldwin and co-workers had reported on the use of azo-anions from N-tert-butyl hydrazones as acyl anion equivalents using the steric protection provided by the bulky tert-butyl group to avoid reaction at the N atom (Scheme 6a), although the reaction with the formaldehyde derivative was not reported.23 Later, the same group also reported on the reactivity of neutral N-monosubstituted hydrazones in thermal ene reactions with highly reactive Michael acceptors such as methyl acrylate and acrylonitrile (Scheme 6b).24 Inspired by these pioneering reports, we envisioned that reactions of neutral formaldehyde N-tert-butyl hydrazone (FTBH) 22 with carbonyl compounds should take place at the azomethine carbon for steric reasons, while the presence of the alkylamino NH group could eventually offer additional opportunities for interactions with bifunctional organocatalysts (Scheme 6c). This strategy proved to be successful for 1,2-additions to carbonyl compounds (formally hetero-carbonyl-ene reactions) as disclosed below.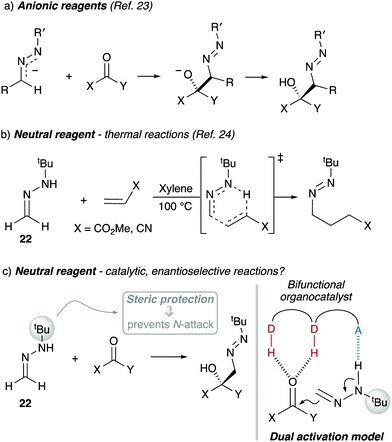 | ||
| Scheme 6 Dual activation strategy with formaldehyde N-tert-butyl hydrazone 22. D–H: H-bond donor, A: H-bond acceptor. | ||
3.2. Addition of FTBH to activated carbonyl compounds
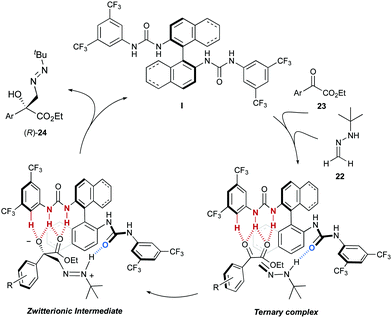
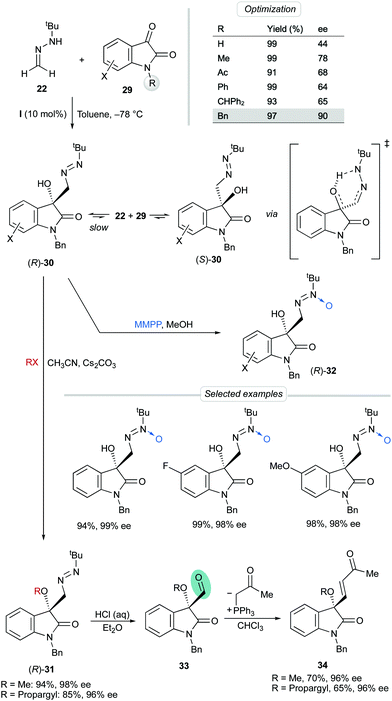
The first asymmetric organocatalytic reactions were planned considering a series of carbonyl substrates containing additional activating functionalities such as a second carbonyl group in α-keto esters25 and isatins (1,2-dicarbonyl systems)26 or a phosphoryl group in α-keto phosphonates.27 In this way, well-defined three-dimensional environments are expected to be created upon engagement with H-bond donor catalysts by multiple-point interactions.Considering the close structural similarity between bis-urea I (I′) and bis-thiourea II (II′) catalysts, the higher hydrogen bond acceptor capability of the oxygen in the carbonyl group of the urea was suggested as the distinct factor. Accordingly, the mechanism and stereochemical model depicted in Scheme 8 was proposed. Hence, a bifunctional behaviour of the catalyst results in the activation of both the hydrazone, by means of a NH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond, and the α-keto ester, through a multiple hydrogen bond network provided by a second urea unit. In this way, a ternary complex is generated, in which the orientation of the bidentate electrophile avoids the aromatic ring being placed in the inner, more crowded region of the catalyst. After nucleophilic attack of the azomethine carbon to the activated carbonyl group and release of the product, the model predicts the formation of the product with the observed (R) absolute configuration at the newly created stereogenic center.
C hydrogen bond, and the α-keto ester, through a multiple hydrogen bond network provided by a second urea unit. In this way, a ternary complex is generated, in which the orientation of the bidentate electrophile avoids the aromatic ring being placed in the inner, more crowded region of the catalyst. After nucleophilic attack of the azomethine carbon to the activated carbonyl group and release of the product, the model predicts the formation of the product with the observed (R) absolute configuration at the newly created stereogenic center.
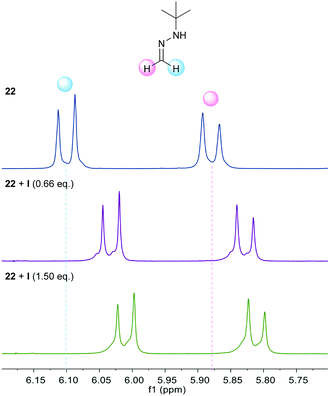
Additional support was obtained from NMR experiments. Thus, 1H NMR spectra recorded for 22 in the presence of increasing amounts of catalyst I showed the signals of the azomethine protons shifted upfield (Δδ up to −0.1 ppm at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
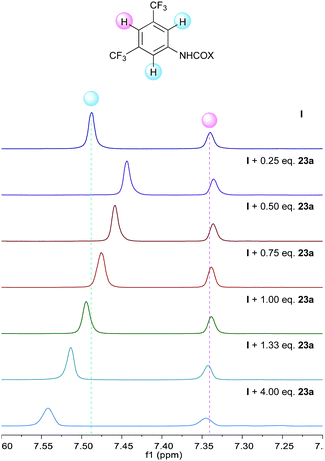
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, Fig. 1), as expected from a higher electron density at the azomethine carbon resulting from the polarization of the N–H bond. Moreover, 1H NMR spectra recorded for I in the presence of increasing amounts of keto ester 23a showed an additional perturbation of the ortho-protons of the catalyst (Fig. 2), suggesting their participation as H-bond donors in a cooperative network.28 Initially, an upfield shift of the signals of these protons was observed upon addition of 0.25 equiv. of keto ester, presumably due to the disruption of inter- and/or intra-molecular self-aggregation of bis-urea catalyst. Progressively, a downfield shift was then observed upon further addition of 23a (Δδ up to 0.1 ppm at a 1
1 ratio, Fig. 1), as expected from a higher electron density at the azomethine carbon resulting from the polarization of the N–H bond. Moreover, 1H NMR spectra recorded for I in the presence of increasing amounts of keto ester 23a showed an additional perturbation of the ortho-protons of the catalyst (Fig. 2), suggesting their participation as H-bond donors in a cooperative network.28 Initially, an upfield shift of the signals of these protons was observed upon addition of 0.25 equiv. of keto ester, presumably due to the disruption of inter- and/or intra-molecular self-aggregation of bis-urea catalyst. Progressively, a downfield shift was then observed upon further addition of 23a (Δδ up to 0.1 ppm at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio), consistent with the establishment of a catalyst–substrate intermolecular H-bonding network.
4 ratio), consistent with the establishment of a catalyst–substrate intermolecular H-bonding network.
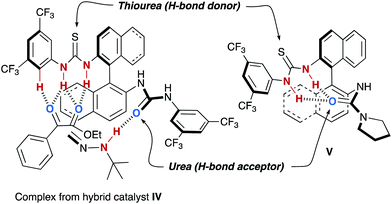
Further support for the proposed bifunctional working model was collected from experiments performed with hybrid thiourea–urea catalyst IV (Fig. 3). According to the proposed mechanism, a single urea moiety is required to activate the reagent 22 and, therefore, IV was expected to imitate the behaviour of I, and not that of II. In fact, this hybrid catalyst afforded azomethyl alcohols with similar enantiomeric excess as I and slightly shorter reaction times, which accounts for the better activation of α-keto esters by the thiourea moiety. However, there is a limitation on the catalyst design: incorporation of a better H-bond acceptor urea, as in pyrrolidine derivative V, results in a lower catalytic activity and the obtention of racemic products. These facts can be explained by the deactivation of the catalyst due to a strong intramolecular H-bonding interaction between the amide carbonyl group, with enhanced H-bond acceptor ability, and the thiourea moiety as a double H-bond donor.29
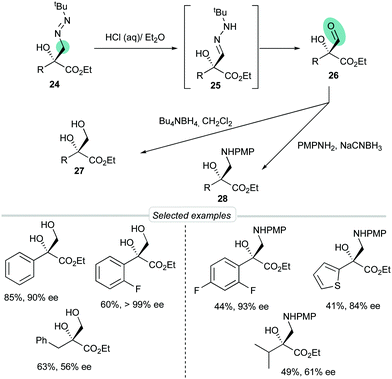
Diazenes 24 are densely functionalized tertiary alcohols which contain the core structures of pharmacologically relevant compounds such as Anisodine, Voriconazole (VFEND®),30 Posaconazole (NOXAFIL®),31 and isoserines (2-substituted α-hydroxy-β-amino acids) which are present in several taxoid-based anticancer agents,32 among others.
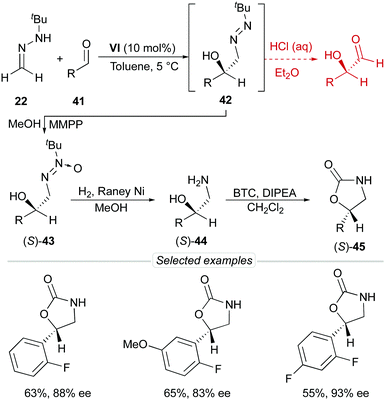
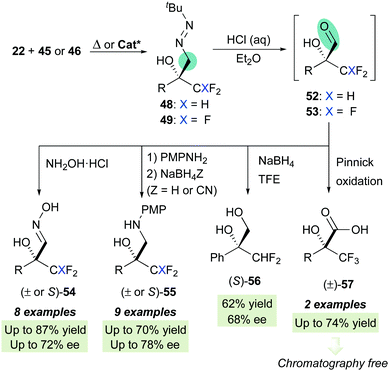
To demonstrate that FTBH 22 can indeed be designated as a formyl anion equivalent, representative diazenes 24 were transformed into aldehydes 26 through a tautomerization (→ 25)/hydrolysis sequence efficiently performed by simple treatment with HCl in a biphasic H2O/Et2O medium (Scheme 9). Crude aldehydes were sensitive products that could not be purified by chromatographic techniques but were isolated with a high degree of purity and could be used directly in subsequent transformations. For example, reductions or reductive aminations afforded diols 27 or α-hydroxy-β-amino esters 28 in good overall yields.
3.3. Addition of FTBH to monocarbonyl compounds
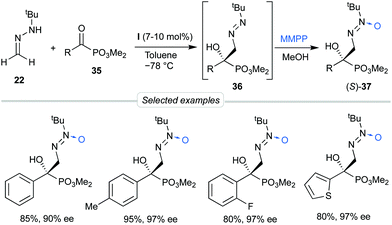
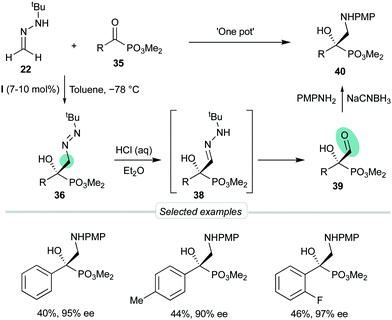
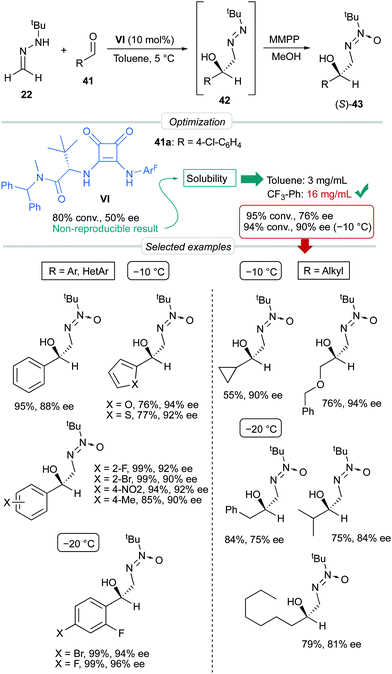
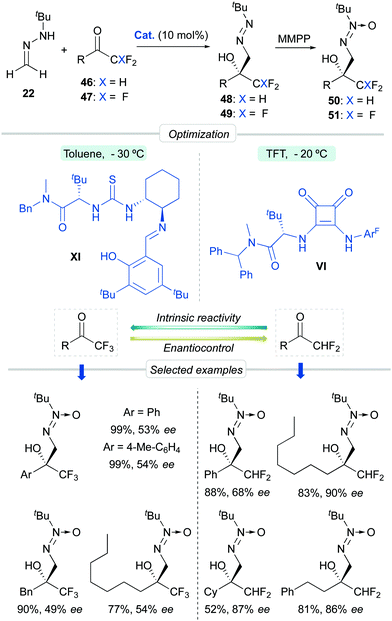
In the next stage, we investigated enantioselective reactions of FTBH with single carbonyl compounds (simple aldehydes36 and fluoromethyl ketones37). These are a priori more challenging substrates for two main reasons: first, the intrinsic electrophilicity of the carbonyl group in these substrates, highly dependent on the substitution patterns, is expected to translate into a significant uncatalyzed, racemic background reaction. Second, the geometry of the catalyst–substrate complexes should be fixed using weak H-bonding interactions with a single acceptor site, in contrast with the above-mentioned precedents.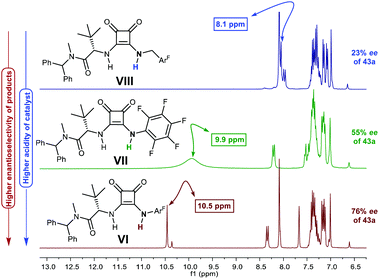 | ||
| Fig. 5 Correlation of catalysts’ H-bond donor ability with enantioselectivity of 43a (1H NMR of aromatic region: 6.5–13.0 ppm shown). | ||
The scope of the reaction was explored employing different aromatic and heteroaromatic aldehydes; the corresponding azoxy compounds 43 were isolated in excellent yields and high enantioselectivities (up to 96% ee). Remarkably, aliphatic aldehydes with representative types of alkyl chains (cyclopropyl, benzyloxymethyl, benzyl, isopropyl, and n-heptyl) were also tolerated.
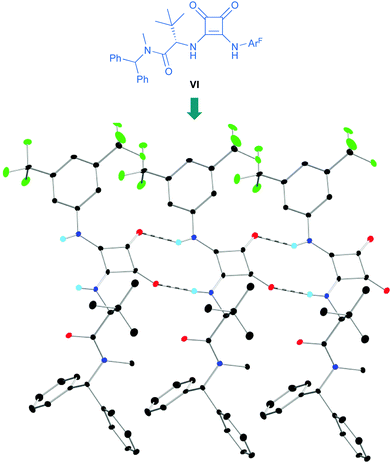
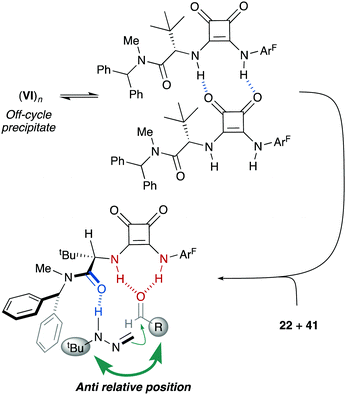
Experimental support for the bifunctional mode of action by squaramide VI was obtained from experiments carried out with monofunctional achiral catalysts IX and X. Thus, these catalysts were evaluated in the reaction between FTBH 22 and 2,6-difluorobenzaldehyde 41b in α,α,α-trifluorotoluene at −10 °C (Scheme 14). The evolution of these control reactions over time (monitored by 19F NMR) showed a much higher catalytic activity of VI (>90% conv. after 10 h) compared to those of IX and X, either acting individually or combined (<30% conv.)39 Consequently, a stereochemical model based on a dual activation of both reagents was proposed, accounting for the observed high enantioselectivities and absolute configuration (Fig. 6). The aldehyde is believed to be activated by the squaramide moiety through H-bonding, while the amide carbonyl group behaves as an efficient H-bond acceptor for the hydrazone NH donor. The activation and the positioning of both reagents drive the preferential approach of azomethine carbon to the Re face of the carbonyl group, minimizing steric repulsions during the C–C bond formation. A slightly negative nonlinear effect suggested a more complex scenario involving insoluble off-cycle catalyst homochiral self-aggregates.40 However, the observed effect was relatively low as reactions become completely homogeneous upon mixing of reagents.
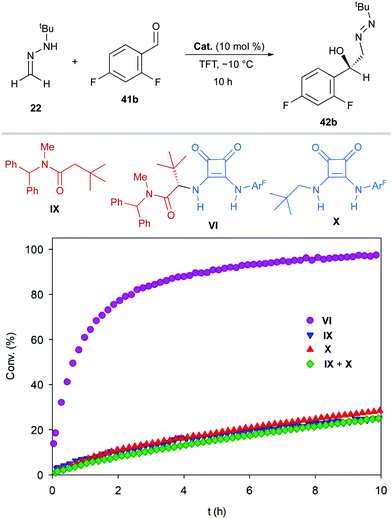 | ||
| Scheme 14 Evolution over time for the addition of FTBH (22) to 2,6-difluorobenzaldehyde (41b) in the presence of bifunctional catalyst VI and monofunctional catalysts IX and/or X. | ||
Despite all efforts, the inherent instability of diazenes 42 made it impossible to obtain α-hydroxy aldehydes in this particular case. Alternatively, azoxy compounds 43 were transformed into free amino alcohols 44 by applying standard hydrogenation conditions [RANEY® Ni, H2 (25–50 bar), room temperature]. This unprecedented transformation involves three consecutive steps [hydrogenolytic N–O bond cleavage (azoxy-to-azo compound), N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond hydrogenation (azo-to-hydrazine reduction) and N–N bond hydrogenolysis (hydrazine-to-amine)] and afforded amino alcohols 44 and derivatives such as oxazolidinones 4541 in good overall yields and without significant erosion of enantioselectivity (Scheme 15).
N bond hydrogenation (azo-to-hydrazine reduction) and N–N bond hydrogenolysis (hydrazine-to-amine)] and afforded amino alcohols 44 and derivatives such as oxazolidinones 4541 in good overall yields and without significant erosion of enantioselectivity (Scheme 15).
4. Conclusions
The combination of formaldehyde tert-butyl hydrazone, as a neutral formyl anion equivalent, with bifunctional H-bond donor/acceptor organocatalysts has emerged as a versatile tool for the asymmetric formylation of carbonyl compounds. The design of a dual activation strategy has been key to successfully perform highly enantioselective nucleophilic additions, formally aza-carbonyl-ene reactions, with a broad scope of substrates including α-keto esters, isatines, α-keto phosphonates, simple aldehydes and fluorinated ketones, efficiently yielding tert-butyl azomethyl carbinols as the primary reaction products. In these reactions, ternary catalyst–reagent–substrate complexes are generated, in which the carbonyl compound of the substrate is activated by multiple H-bond interactions (with a urea/thiourea/squaramide moiety), while the nucleophilicity of the hydrazone reagent is enhanced by an additional H-bonding interaction with the basic oxygen atom of an urea or amide group. In most cases, the formylation procedure is efficiently completed after a simple ‘one-pot’ transformation of the obtained diazenes into the targeted aldehydes via tautomerization followed by hydrolytic cleavage of the hydrazone intermediates. Moreover, a variety of other densely functionalized chiral building blocks can be also obtained after simple functional group transformations. Despite the high reactivity observed for some substrates, none of the catalysts used allowed to expand the reactivity to N-tert-butylhydrazones from higher aldehydes.43 Future investigations based in alternative catalysts and/or activation strategies, therefore, will be needed to overcome this limitation and develop a more general method for the asymmetric acylation of carbonyl compounds. Moreover, the implementation of some of these approaches in target-oriented synthesis might be also explored for accessing on-demand biologically active targets.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish MINECO (CTQ2016-76908-C2-1-P, CTQ2016-76908-C2-2-P), European FEDER funds and the Junta de Andalucía (Grants P18-FR-3531 and US-1262867).Notes and references
- (a) D. Seebach, Angew. Chem., Int. Ed. Engl., 1979, 18, 239 CrossRef; (b) T. A. Hase, Umpoled Synthons: A survey of Sources and Uses in Synthesis, Wiley Interscience, New York, 1987 Search PubMed.
- (a) Review: J. Johnson, Angew. Chem., Int. Ed., 2004, 43, 1326 CrossRef CAS PubMed . See also: ; (b) D. Enders and U. Kallfass, Angew. Chem., Int. Ed., 2002, 41, 1743 CrossRef CAS; (c) S. E. Denmark and T. W. Wilson, Nat. Chem., 2010, 2, 937 CrossRef CAS PubMed; (d) D. Enders, A. Grossmann, J. Fronert and G. Raabe, Chem. Commun., 2010, 46, 6282 RSC.
- Reviews: (a) D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534 CrossRef CAS PubMed; (b) M. Christmann, Angew. Chem., Int. Ed., 2005, 44, 2632 CrossRef CAS PubMed; (c) J. S. Johnson, Angew. Chem., Int. Ed., 2004, 43, 1326 CrossRef CAS PubMed; (d) X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511 RSC.
- T. Matsumoto, H. Yamamoto and S. Inoue, J. Am. Chem. Soc., 1984, 106, 4829 CrossRef CAS.
- A. Kirschning, C. Kujat, S. Luiken and E. Schaumann, Eur. J. Org. Chem., 2007, 2387 CrossRef CAS and references therein.
- L. Colombo, C. Gennari and C. Scolastico, J. Chem. Soc., Chem. Commun., 1979, 591 RSC.
- B. Delouvrié, F. Nájera, L. Fensterbank and M. Malacria, J. Organomet. Chem., 2002, 643–644, 130 CrossRef.
- R. E. Gawley, S. A. Campagna, M. Santiago and T. Ren, Tetrahedron: Asymmetry, 2002, 13, 29 CrossRef CAS.
- (a) C. Gaul and D. Seebach, Org. Lett., 2000, 2, 1501 CrossRef CAS PubMed; (b) C. Gaul, K. Schärer and D. Seebach, J. Org. Chem., 2001, 66, 3059 CrossRef CAS PubMed.
- S. Nakamura, Y. Ito, L. Wang and T. Toru, J. Org. Chem., 2004, 69, 1581 CrossRef CAS PubMed.
- (a) L. Wang, S. Nakamura and T. Toru, Org. Biomol. Chem., 2004, 2, 2168 RSC; (b) L. Wang, S. Nakamura, Y. Ito and T. Toru, Tetrahedron: Asymmetry, 2004, 15, 3059 CrossRef CAS.
- (a) J. M. Lassaletta and R. Fernández, Synlett, 2000, 1228 Search PubMed; (b) R. Brehme, D. Enders, R. Fernández and J. M. Lassaletta, Eur. J. Org. Chem., 2007, 5629 CrossRef CAS . The nucleophilicity of formaldehyde N,N-dialkylhydrazones has been measured and correlated with their ambident reactivity: ; (c) M. Biplab, T. Konstantin and M. Herbert, Angew. Chem., Int. Ed., 2013, 52, 11900 CrossRef PubMed.
- D. Monge and R. Fernández, (S)-2-Methoxymethyl-1-methylideneamino-pyrrolidine, Encyclopedia of Reagents for Organic Synthesis, John Wiley and Sons, 2019 Search PubMed.
- (a) J. M. Lassaletta, R. Fernández, E. Martín-Zamora and C. Pareja, Tetrahedron Lett., 1996, 37, 5787 CrossRef CAS; (b) R. Fernández, E. Martín-Zamora, C. Pareja and J. M. Lassaletta, J. Org. Chem., 2001, 66, 5201 CrossRef PubMed.
- R. Fernández, E. Martín-Zamora, C. Pareja, M. Alcarazo, J. Martín and J. M. Lassaletta, Synlett, 2001, 1158 CrossRef.
- (a) R. Fernández, E. Martín-Zamora, C. Pareja, J. Vázquez, E. Díez, A. Monge and J. M. Lassaletta, Angew. Chem., Int. Ed., 1998, 37, 3428 CrossRef; (b) C. Pareja, E. Martín-Zamora, R. Fernández and J. M. Lassaletta, J. Org. Chem., 1999, 64, 8846 CrossRef CAS PubMed.
- D. Enders, L. Wortmann and R. Peters, Acc. Chem. Res., 2000, 33, 157 CrossRef CAS PubMed.
- R. Fernández, C. Gasch, J. M. Lassaletta, J. M. Llera and J. Vázquez, Tetrahedron Lett., 1993, 34, 141 CrossRef.
- Some exceptions: (a) D. Monge, E. Martín-Zamora, J. Vázquez, M. Alcarazo, E. Álvarez, R. Fernández and J. M. Lassaletta, Org. Lett., 2007, 9, 2867 CrossRef CAS PubMed; (b) S. Breitler and E. M. Carreira, J. Am. Chem. Soc., 2015, 137, 5296 CrossRef CAS PubMed.
- Hydrazones in asymmetric organocatalysis: M. G. Retamosa, E. Matador, D. Monge, J. M. Lassaletta and R. Fernández, Chem. – Eur. J., 2016, 22, 13430 CrossRef CAS PubMed.
- (a) R. P. Herrera, D. Monge, E. Martín-Zamora, R. Fernández and J. M. Lassaletta, Org. Lett., 2007, 9, 3303 CrossRef CAS PubMed . For selected previous diastereoselective Michael additions of chiral formaldehyde N,N-dialkylhydrazones, see: ; (b) J. M. Lassaletta, R. Fernández, E. Martín-Zamora and E. Díez, J. Am. Chem. Soc., 1996, 118, 7002 CrossRef CAS; (c) J. Vázquez, A. Prieto, R. Fernández, D. Enders and J. M. Lassaletta, Chem. Commun., 2002, 498 RSC; (d) J. Vázquez, E. Cristea, E. Díez, J. M. Lassaletta, A. Prieto and R. Fernández, Tetrahedron, 2005, 61, 4115 CrossRef.
- (a) D. J. Dixon and A. L. Tillman, Synlett, 2005, 2635 CrossRef CAS; (b) M. Rueping, E. Sugiono, T. Theissmann, A. Kuenkel, A. Köckritz, A. Pews-Davtyan, N. Nemati and M. Beller, Org. Lett., 2007, 9, 1065 CrossRef CAS PubMed . For examples of 1,2-additions of hydrazones from higher aldehydes, see: ; (c) T. Hashimoto, M. Hirose and K. Maruoka, J. Am. Chem. Soc., 2008, 130, 7556 CrossRef CAS PubMed; (d) T. Hashimoto, H. Kimura and K. Maruoka, Angew. Chem., Int. Ed., 2010, 49, 6844 CrossRef CAS PubMed.
- J. E. Baldwin, R. M. Adlington, J. C. Bottaro, J. N. Kolhe, M. W. D. Perry and A. U. Jain, Tetrahedron, 1986, 42, 4223 CrossRef CAS.
- J. E. Baldwin, R. M. Adlington, A. U. Jain, J. N. Kolhe and M. W. D. Perry, Tetrahedron, 1986, 42, 4247 CrossRef CAS.
- (a) A. M. Crespo-Peña, D. Monge, E. Martín-Zamora, E. Álvarez, R. Fernández and J. M. Lassaletta, J. Am. Chem. Soc., 2012, 134, 12912 CrossRef PubMed; (b) J. A. Carmona, G. de Gonzalo, I. Serrano, A. M. Crespo-Peña, M. Šimek, D. Monge, R. Fernández and J. M. Lassaletta, Org. Biomol. Chem., 2017, 15, 2993 RSC.
- D. Monge, A. M. Crespo-Peña, E. Martín-Zamora, E. Álvarez, R. Fernández and J. M. Lassaletta, Chem. – Eur. J., 2013, 19, 8421 CrossRef CAS PubMed.
- I. Serrano, D. Monge, E. Álvarez, R. Fernández and J. M. Lassaletta, Chem. Commun., 2015, 51, 4077 RSC.
- For a related study showing the participation of the ortho CH bonds of the 3,5-bis(trifluoromethyl)phenyl group as donors H-bond networks, see: Z. Zhang, K. M. Lippert, H. Hausmann, M. Kotke and P. R. Schreiner, J. Org. Chem., 2011, 76, 9764 CrossRef CAS PubMed.
- For a closely related structure in solid state, see: R. Holakovský, M. Pojarová, M. Dusek, J. Cejka and I. Císarová, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, o384 CrossRef PubMed.
- K. Tamura, M. Furutachi, N. Kumagai and M. Shibasaki, J. Org. Chem., 2013, 78, 11396 CrossRef CAS PubMed and references therein.
- H. K. Munayyer, P. A. Mann, A. S. Chau, T. Yarosh-Tomaine, J. R. Greene, R. S. Hare, L. Heimark, R. E. Palermo, D. Loebenberg and P. M. McNicholas, Antimicrob. Agents Chemother., 2004, 48, 3690 CrossRef CAS PubMed.
- (a) I. Ojima, C. M. Sun, M. Zucco, Y. H. Park, O. Duclo and S. Kuduk, Tetrahedron Lett., 1993, 34, 4149 CrossRef CAS; (b) I. Ojima, T. Wang and F. Delaloge, Tetrahedron Lett., 1998, 39, 3663 CrossRef CAS; (c) G. Cardillo, L. Gentilucci, A. Tolomelli and C. Tomasini, J. Org. Chem., 1998, 63, 2351 CrossRef CAS; (d) Y. Takeda, T. Yoshino, K. Uoto, J. Chiba, T. Ishiyama, M. Iwahana, T. Jimbo, N. Tanaka, H. Terasawa and T. Soga, Bioorg. Med. Chem. Lett., 2003, 13, 185 CrossRef CAS PubMed.
- S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20 CrossRef CAS.
- Selected examples of bioactive azoxy compounds: (a) The antimicrobial and cytotoxic elaiomycins: L. Ding, B. L. T. Ndejouong, A. Maier, H. H. Fiebig and C. Hertweck, J. Nat. Prod., 2012, 75, 1729 CrossRef CAS PubMed; (b) The antibiotic valanimycin: R. P. Garg, X. L. L. Qian, L. B. Alemany, S. Moran and R. J. Parry, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 6543 CrossRef CAS PubMed; (c) Novel antifungal antibiotics maniwamycins A and B: M. Nakayama, Y. Takahashi, H. Itoh, K. Kamiya, M. Shiratsuchi and G. Otani, J. Antibiot., 1989, 42, 1535 CrossRef CAS PubMed.
- (a) For a review on naturally occurring β-amino-α-hydroxyphosphonic acid derivatives, see: P. Mastałerz and P. Kafarski, in Aminophosphonic and Aminophosphinic Acids-Chemistry and Biological Activity, ed. V. P. Kukhar and H. R. Hudson, John Wiley, Chichester, 2000, pp 1–31 Search PubMed; (b) D. V. Patel, K. Rielly-Gauvin, D. E. Ryono, C. A. Free, W. L. Rogers, S. A. Smith, J. M. DeForrest, R. S. Oehl and E. W. Petrillo, J. Med. Chem., 1995, 38, 4557 CrossRef CAS PubMed.
- E. Matador, M. G. Retamosa, D. Monge, J. Iglesias-Sigüenza, R. Fernández and J. M. Lassaletta, Chem. – Eur. J., 2018, 24, 6854 CrossRef CAS PubMed.
- E. Matador, M. G. Retamosa, A. Jiménez-Sánchez, D. Monge, R. Fernández and J. M. Lassaletta, Eur. J. Org. Chem., 2019, 130 CrossRef CAS.
- See: (a) A. Portell, R. Barbas, D. Braga, M. Polito, C. Puigjanerand and R. Prohens, CrystEngComm, 2009, 11, 52 RSC; (b) A. Portell, M. Font-Bardia, A. Bauzá, A. Frontera and R. Prohens, Cryst. Growth Des., 2014, 14, 2578 CrossRef; (c) A. Portell, M. Bardia-Font, A. Bauzá, A. Frontera and R. Prohens, CrystEngComm, 2016, 18, 6437 RSC.
- For a discussion on multiple vs multifunctional catalysis, see: S. Piovesana, D. M. S. Schietroma and M. Bella, Angew. Chem., Int. Ed., 2011, 50, 6216 CrossRef CAS PubMed.
- In contrast, Jacobsen and co-workers described a positive non-linear effect due to the presence of soluble off-cycle catalysts homochiral self-aggregates for related amide-thioureas in different contexts. See: (a) D. D. Ford, D. Lehnherr, C. R. Kennedy and E. N. Jacobsen, J. Am. Chem. Soc., 2016, 138, 7860 CrossRef CAS PubMed; (b) D. Ford, D. Lehnherr, C. R. Kennedy and E. N. Jacobsen, ACS Catal., 2016, 6, 4616 CrossRef CAS PubMed.
- Oxazolidinones in medicinal chemistry: (a) T. A. Mukhtar and G. D. Wright, Chem. Rev., 2005, 105, 529 CrossRef CAS PubMed; (b) M. R. Barbachyn and C. W. Ford, Angew. Chem., Int. Ed., 2003, 42, 2010 CrossRef CAS PubMed; (c) H. Kakeya, M. Morishita, K. Kobinata, M. Osono, M. Ishizuka and J. Osada, J. Antibiot., 1998, 51, 1126 CrossRef CAS PubMed; (d) M. F. Gordeev and Y. Y. Zhengyu, J. Med. Chem., 2014, 57, 4487 CrossRef CAS PubMed.
- E. Matador, D. Monge, R. Fernández and J. M. Lassaletta, Green Chem., 2016, 18, 4042 RSC.
- Recent example on the asymmetric 1,2-addition of N-tert-butylhydrazones to imines employing Brønsted-acid catalysis: Y. Wang, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2017, 56, 5612 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to the memory of Professor Kilian Muñiz. |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |