Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cell-free protein synthesis in hydrogel materials†
Colette J.
Whitfield
 a,
Alice M.
Banks
a,
Alice M.
Banks
 a,
Gema
Dura
a,
Gema
Dura
 a,
John
Love
a,
John
Love
 b,
Jonathan E.
Fieldsend
b,
Jonathan E.
Fieldsend
 c,
Sarah A.
Goodchild
d,
David A.
Fulton
c,
Sarah A.
Goodchild
d,
David A.
Fulton
 a and
Thomas P.
Howard
a and
Thomas P.
Howard
 *a
*a
aSchool of Natural and Environmental Sciences, Faculty of Science, Agriculture and Engineering, Newcastle University, Newcastle, UK. E-mail: thomas.howard@newcastle.ac.uk
bBiosciences, College of Life and Environmental Sciences, University of Exeter, Exeter, UK
cComputer Science, College of Engineering, Mathematics and Physical Sciences, University of Exeter, UK
dDefence Science and Technology Laboratory, Porton Down, Wiltshire, UK
First published on 27th May 2020
Abstract
We report a method for embedding cell-free protein synthesis reactions in macro-scale hydrogel materials without a free liquid phase. This paper focuses on methods of preparation for a variety of hydrogels and an investigation of the impact that the hydrogel material has on cell-free protein synthesis.
Cell-free protein synthesis (CFPS) uses cellular transcriptional and translational machinery to synthesise proteins outside the living cell.1 CFPS systems have shown potential as diagnostic tools in the detection of Zika and Ebola viruses,2,3 as well as for detection of metabolically important molecules such as hippuric acid and cocaine.4 CFPS is typically performed in liquid reactions in vitro, though there have been recent developments in the use of cell-free protein synthesis on paper supports and with microgels fabricated from DNA,5 agarose,6 clay,7 hyaluronan,8 polyacrylamide,9 fibrin and PEG-peptide.10 In each instance microgels are prepared and incubated within CFPS reaction mixtures,5,6,8,9 or hydrogel polymers form around an active liquid CFPS reaction.7,10 As such we cannot exclude the possibility that some aspects of the CFPS reaction (i.e. transcription or translation) occur in the reaction solution outside the gel and protein products diffuse into the gel. Alternatively, protein synthesis may occur before the gel has formed, producing a functional enzyme in liquid phase. Subsequent gel polymerisation may be the result of the enzyme product rather than active CFPS reactions.
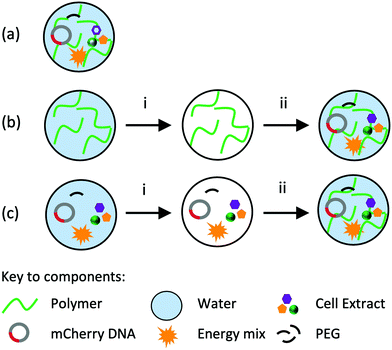
Importantly, hydrogels have valuable properties in their own right. They act as pastes,11 lubricants12 and adhesives13 or in the provision of structural support for regenerative cell growth.14 Considering the broad applications of hydrogels and the functionality of CFPS, we hypothesise that hydrogel matrices may act as a physical chassis for CFPS – allowing CFPS to move from a predominantly in vitro application into a physical, biologically activated material. If CFPS reactions are to be deployed in a material chassis, it is critical that the reactions occur within the material and without a separate liquid phase, and that we understand the impact hydrogels themselves may have on CFPS. Moreover, it is also important to explore how broadly suitable macro-scale hydrogels are as physical chassis for CFPS. Previous studies conducting CFPS in hydrogels typically use microgel systems ranging from 1 to 400 μm in size.5–10 Here, we describe methods of embedding cell-free cellular components capable of performing protein synthesis in hydrogels (Scheme 1). Using these approaches, we demonstrate that CFPS can be performed throughout a range of hydrogel materials synthesised at macro-scale (2–20 mm) and in the absence of an external liquid phase. Moreover, the methods allow us to probe the physical effect that hydrogels can have on the performance of CFPS.
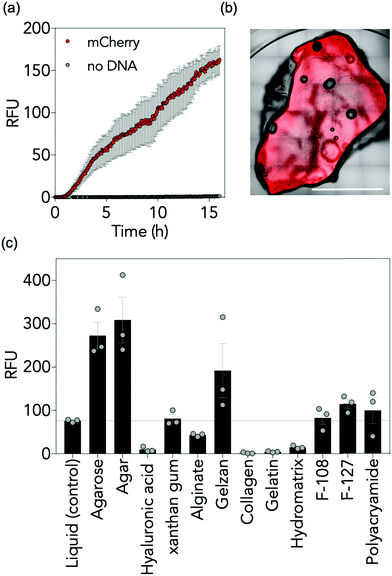
First, we established gene expression of mCherry in agarose gels using method (a) (Scheme 1 and Fig. 1a). Briefly, gels were prepared by the addition of molten (60 °C) 3% agarose to cell-free reaction components in the appropriate cast. Gels were allowed to set for 30 min at room temperature before incubation at 37 °C. All reaction components were situated within the hydrogel polymer network; there was no external liquid observed in the system. We monitored the appearance of mCherry fluorescence over 16 h using spectroscopy and confirmed fluorescence within the gels using confocal microscopy. Our data indicated that mCherry fluorescence increased throughout the time period, and was detected and located evenly throughout the material (Fig. 1a, b and ESI,† Fig. S1). As the fluorescence increase was continuous, we conclude that CF components are stable in gels at 37 °C for 16 h.
With an analytical system established, we next determined whether CFPS is compatible with a broad range of hydrogel materials. Three fabrication methods were used to integrate CFPS reaction components within the hydrogels in the absence of an external liquid phase (Scheme 1). For method (a), each component was prepared fresh, i.e. hydrogel components and freshly prepared cell-free components were added together and allowed to set before analysis. In some cases, cell-free components were added directly to solid polymer powders whereas others were added to concentrated liquid hydrogels. In method (b), the gel was prepared and allowed to set in the appropriate vessel, for example a 384-well plate or Petri dish, freeze-dried and reconstituted with freshly prepared cell-free reaction components. The gel was then allowed to re-form for 30 min before instigating further experimental procedures. The appropriate volume to reconstitute each hydrogel can be calculated from the % reconstitution in Table S1, ESI.† In method (c), the CFPS reaction components were combined on ice, freeze-dried, and reconstituted with liquid hydrogel. Again, the gel was allowed to reform for 30 min prior to further work. Rheological analysis of each material was performed to confirm that all materials were gels (Fig. S2 and Table S2, ESI†). To ascertain active CFPS in these gels, we expressed the mCherry reporter protein. We analysed expression in 12 gels prepared by each possible method with a variety of different structural and chemical characteristics, including entangled polymers, micellar aggregates and covalently crosslinked materials. A variety of polymer weight to volume (w/v) ratios were examined to determine the optimum concentration for fluorescent output, see Fig. S3 and Table S3, ESI.† For some hydrogels, all methods were appropriate, whilst for others, only one method was feasible and may have required additional steps, such as dialysis. We monitored the fluorescence output for mCherry and compared the maximum fluorescence observed over a 16 h period. Two controls were employed: a positive control (CFPS of mCherry in liquid phase) and a negative control (CFPS reaction mixture lacking a DNA template to ensure all fluorescence detected could be attributed to mCherry protein synthesis, ESI,† Fig. S3). Overall, our data indicated that the ability to perform CFPS in hydrogels, though variable, is possible in different matrices (Fig. 1c, ESI,† Table S3 and Fig. S3). Of the 12 gels tested, six were polysaccharide-based hydrogels: agarose, agar, xanthan gum, hyaluronic acid, Gelzan™ and alginate. These gels feature extensive, non-covalent linkages between polymer chains, formed by hydrogen bonding and van der Waals forces (agarose, agar, xanthan and hyaluronic acid) or divalent ionic bridges (Mg2+ for Gelzan™ and Ca2+ for alginate). For agarose, agar, xanthan, and Gelzan™ the fluorescence detected was equivalent to, or up to 400% greater than the fluorescence observed when CFPS was conducted in liquid phase. For alginate and hyaluronic acid in contrast, there was a reduction in fluorescence of between 13 and 57% respectively compared to the control. Three amino acid-based hydrogels were also assessed: one protein hydrogel (collagen) and two peptide hydrogels (gelatin and HydroMatrix™). These gels are formed through hydrophobic interactions, π–π stacking, hydrogen bonding and electrostatic interactions between polymers.15 For each of the three peptide-based hydrogels CFPS was observed above baseline but fluorescence signals were low, reaching between 6 and 43% of that observed in the positive control. Pluronic acid F-108 and F-127 are poloxamer gels where individual micelles aggregate to form a gel. For these gels, fluorescence was equivalent to, or up to, 120% of the liquid phase control. Finally, an examination of CFPS in a polyacrylamide gel, a covalently crosslinked network formed from the polymerisation of acrylamide and bis-acrylamide, revealed that CFPS-fluorescence was 110% of that observed in the liquid phase control reaction. CFPS is therefore possible in a range of hydrogel materials and, in some instances, performing the reaction in a hydrogel chassis results in an increase in the fluorescent output of the reaction. It follows that choice of hydrogel chassis for CFPS is not restrictive, and that a gel can be selected that is appropriate to the proposed end-use.
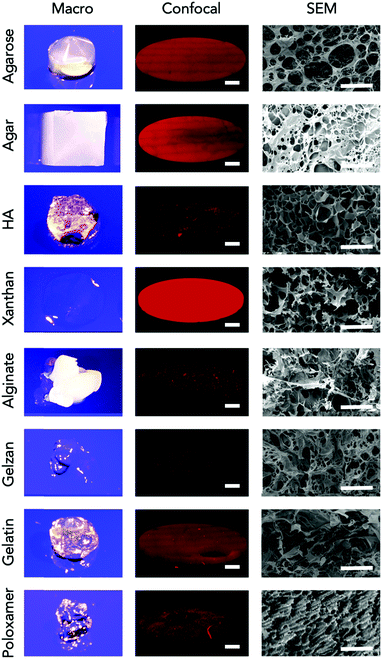
To confirm that gene expression occurs within the material, confocal microscopy was used to observe mCherry fluorescence throughout the gels (Fig. 2 and Fig. S4, ESI†). Gels demonstrating equivalent or increased fluorescence compared to control reactions (agarose, agar and xanthan gels) displayed homogenous mCherry-fluorescence throughout the gel. Others, in which cell-free production of mCherry was detected but reduced (e.g. hyaluronic acid) demonstrated heterogeneous patterns of fluorescence. Scanning Electron Microscopy (SEM) (Fig. 2) of a selection of gels suggests that large, open pores correspond with homogenous patterns of gene expression in comparison to those with smaller, or more irregular shaped pores. Additionally, the presence of homogenous and regular pore sizes also corresponds with higher fluorescence outputs. We therefore hypothesised that the ability of reagents to diffuse through the material would significantly impact on CFPS function. To test this, we examined the diffusion of fluorescein (a small molecule fluorophore) at different agarose w/v ratios. We observed that an increase in pore size correlated with an increase in observed diffusion (Fig. S5, ESI†) while increasing w/v ratio and decreasing the pore size correlated with a decrease in diffusion ability. This data along with the confocal and SEM observations support the view that gels with larger, more open pores allow greater diffusion and therefore a higher level of protein synthesis.
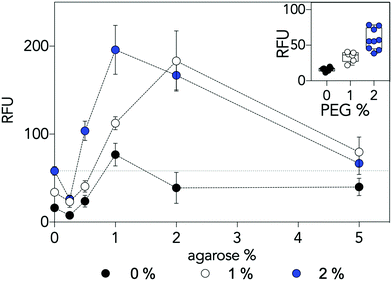
Macromolecular crowding is an established mechanism that positively influences CFPS reactions.16 Macromolecular molecules (commonly polyethylene glycol (PEG) in CFPS reactions) are present at a high concentration resulting in an increase of local effective concentration for the reaction's substrates. Given that diffusion is not the sole factor influencing CFPS output, we hypothesised that the hydrogel network may also act as a macromolecular crowder. To test this, we assayed CFPS in agarose gels without the crowding agent PEG and with PEG at half the standard concentration (1%). Reactions in the absence of agarose and lacking PEG demonstrated reductions in CFPS dependent fluorescence of 72% while those at half standard PEG concentrations demonstrated reductions of 42% (Fig. 3 inset). The data indicated that 1% agarose gels without PEG maintained fluorescence values equivalent to those observed in standard liquid phase reactions with 2% PEG. Moreover, reductions in CFPS output observed when PEG concentrations are reduced by half are more than recovered by performing the reactions in 1 or 2% agarose gels (Fig. 3). This data supports the hypothesis that the hydrogel matrix acts as a structural crowding agent as well as a physical support.
Here, we have demonstrated the fabrication and function of CFPS reactions in a range of hydrogel materials in the absence of an external liquid phase. CFPS reactions were possible inside polysaccharide, proteinaceous, micellar and covalently crosslinked hydrogels. Moreover, we have shown internal gel diffusion and the ability of gels to act as a structural molecular crowding agent impacts CFPS reaction efficacy. Our data supports the view that the range of hydrogel polymers compatible with CFPS is broad, not narrow, meaning that the selection of hydrogel can be appropriate to its desired use. Importantly, though we see an apparent benefit in increased protein production in some gels, the broad application across a range of gels means that even with decreases in CFPS performance, if the gel provides a functional or structural benefit to the designer (for example biocompatibility or adhesive properties) then there may be a permissible trade-off. Some loss of CFPS capacity may be tolerated for a gain of function that a particular gel may offer. However, while it is likely that the choice of reporter can be expanded to other fluorescent and chromophore reporter proteins, the key to broadening the applications of this approach will be to express and detect non-reporter proteins, and to express multiple proteins simultaneously, whose combined action enhances hydrogel functionality. Challenges in expressing non-reporter proteins will likely lie in the ability to assay functional protein synthesised within a gel matrix. Despite these difficulties, we propose that implementing cell-free gene expression in hydrogel materials will allow their deployment as physical biologically activated standalone materials.
The authors greatly acknowledge the support of the Engineering and Physical Sciences Research Council – Defence Science and Technology Laboratories award EP/N026683/1 (CJW, AMB, JL, JEF, DAF, TPH) and the Biotechnology and Biological Sciences Research Council award BB/M018318/1 (GD, DAF). Data supporting this publication is openly available through a CC0 1.0 Universal (CC0 1.0) Public Domain Dedication at: https://doi.org/10.25405/data.ncl.12349352.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. E. Hodgman and M. C. Jewett, Metab. Eng., 2012, 14, 261 CrossRef CAS PubMed.
- K. Pardee, A. A. Green, M. K. Takahashi, D. Braff, G. Lambert, J. W. Lee, T. Ferrante, D. Ma, N. Donghia, M. Fan, N. M. Daringer, I. Bosch, D. M. Dudley, D. H. O'Connor, L. Gehrke and J. J. Collins, Cell, 2016, 165, 1255 CrossRef CAS PubMed.
- K. Pardee, A. A. Green, T. Ferrante, D. E. Cameron, A. D. Keyser, P. Yin and J. J. Collins, Cell, 2014, 159, 940 CrossRef CAS.
- P. L. Voyvodic, A. Pandi, M. Koch, I. Conejero, E. Valjent, P. Courtet, E. Renard, J.-L. Faulon and J. Bonnet, Nat. Commun., 2019, 10, 1697 CrossRef.
- (a) J. S. Kahn, R. C. H. Ruiz, S. Sureka, S. Peng, T. L. Derrien, D. An and D. Luo, Biomacromolecules, 2016, 17, 2019 CrossRef CAS PubMed; (b) N. Park, S. H. Um, H. Funabashi, J. Xu and D. Luo, Nat. Mater., 2009, 8, 432 CrossRef CAS PubMed.
- J. Y. Byun, K. H. Lee, K. Y. Lee, M. G. Kim and D. M. Kim, Lab Chip, 2013, 13, 886 RSC.
- D. Yang, S. Peng, M. R. Hartman, T. Gupton-Campolongo, E. J. Rice, A. K. Chang, Z. Gu, G. Q. Lu and D. Luo, Sci. Rep., 2013, 3, 3165 CrossRef PubMed.
- T. Heida, T. Köhler, A. Kaufmann, M. J. Männel and J. Thiele, ChemSystemsChem, 2020, 2, e1900058 CAS.
- X. Zhou, H. Wu, M. Cui, S. N. Lai and B. Zheng, Chem. Sci., 2018, 9, 4275 RSC.
- A. Huang, P. Q. Nguyen, J. C. Stark, M. K. Takahashi, N. Donghia, T. Ferrante, A. J. Dy, K. J. Hsu, R. S. Dubner, K. Pardee, M. C. Jewett and J. J. Collins, Sci. Adv., 2018, 4, 5105 CrossRef PubMed.
- F. Yoshii, L. Zhao, R. A. Wach, N. Nagasawa, H. Mitomo and T. Kume, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 208, 320 CrossRef CAS.
- M. E. Freeman, M. J. Furey, B. J. Love and J. M. Hampton, Wear, 2000, 241, 129 CrossRef CAS.
- C. E. Brubaker and P. B. Messersmith, Biomacromolecules, 2011, 12, 4326 CrossRef CAS PubMed.
- A. C. Jen, M. C. Wake and A. G. Mikos, Biotechnol. Bioeng., 1996, 50, 357 CrossRef CAS PubMed.
- A. Dasgupta, J. H. Mondal and D. Das, RSC Adv., 2013, 3, 9117 RSC.
- (a) B. R. Fritz, O. K. Jamil and M. C. Jewett, Nucleic Acids Res., 2015, 43, 4774 CrossRef CAS PubMed; (b) C. Tan, S. Saurabh, M. P. Bruchez, R. Schwartz and P. Leduc, Nat. Nanotechnol., 2013, 8, 602 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, methods, supplementary data and hydrogel characterisation. See DOI: 10.1039/d0cc02582h |
| This journal is © The Royal Society of Chemistry 2020 |