Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-induced molecular rotation triggers on-demand release from liposomes†
Laís
Ribovski
 ab,
Qihui
Zhou
ab,
Qihui
Zhou
 c,
Jiawen
Chen
d,
Ben L.
Feringa
c,
Jiawen
Chen
d,
Ben L.
Feringa
 d,
Patrick
van Rijn
d,
Patrick
van Rijn
 *a and
Inge S.
Zuhorn
*a and
Inge S.
Zuhorn
 *a
*a
aUniversity of Groningen, University Medical Center Groningen, Department of Biomedical Engineering, A. Deusinglaan 1, 9713 AV Groningen, The Netherlands. E-mail: p.van.rijn@umcg.nl; i.zuhorn@umcg.nl
bNanomedicine and Nanotoxicology Group, Physics Institute of São Carlos, University of São Paulo, CP 369, 13560-970 São Carlos, SP, Brazil
cInstitute for Translational Medicine, Department of Periodontology, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao 266021, China
dCenter for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747AG Groningen, The Netherlands
First published on 1st July 2020
Abstract
Controllable molecular release from delivery vehicles is essential to successfully reduce drug toxicity and improve therapeutic efficacy. Light-powered hydrophobic molecular motors were therefore incorporated in liposomes to use molecular rotation to facilitate on-demand release. The extent of the release was precisely controlled by irradiation times, providing a simple yet sophisticated responsive molecular nanocarrier.
Within the field of nanomedicine, specialized approaches to transport pharmaceutically active compounds to target sites by means of nanostructures is one of the main goals.1 The use of specialized nanocarriers (NCs) are considered to be highly promising in treating various diseases including combating infections, inflammation, fibrosis, and cancer.2,3 Many nanoparticle systems have been developed over the years for diagnosis and therapy of diseases, which includes solid inorganic nanoparticles, polymeric nanoparticles such as micelles and polymersomes, protein nanoparticles, and lipid-based nanoparticles such as liposomes and lipid nanoparticles (LNPs).2,4–8 A key aspect of NCs is not only to accommodate the drugs but particularly to release them on demand in order to increase local drug concentrations to achieve therapeutic effectiveness, while preventing side effects.5
For on demand drug release from NCs, external triggers or local factors are often envisioned. Local factors that can be exploited are e.g. a change in pH (such as in tumor tissues)9 or alterations in temperature and pH due to inflammation of the tissue.10 Many stimuli-responsive systems that have been developed are polymer-based, because of the good control over their composition, which is necessary to fine-tune their response to specific stimuli and highly promising drug delivery systems have been developed based on these polymers.11,12
Lipid-based systems are also extremely attractive for triggered release because of ease of preparation, flexibility of design, low immune response, and capability of containing large payloads which facilitates clinical translation.13 Liposomes are often employed for delivery of genetic material (gene delivery),14–16 anti-cancer drugs,17,18 hormones,19 and for imaging purposes.20,21 The lipid-delivery systems can also rely on controlled/triggered-release taking advantage of local factors of the microenvironment in diseased tissue, which is not desirable in case of inter-patient and intra-patient heterogeneity. Hence, triggers that are not purely related to the local environment are being used in the development of nanocarriers, including magnetic fields,22 ultrasound,23 and light.24
Particularly the use of photo-responsive delivery approaches is highly desirable, as these will enable on demand release. Photo-responsive polymersomes have been developed to release molecular payloads,25 including light-triggered nitric oxide release for corneal wound healing.26 Light-dependent release has the disadvantage of potential phototoxicity,27 but if the system allows short enough exposure, phototoxicity can be prevented or reduced. Another disadvantage is that most release mechanisms induce membrane destabilization and membrane stability cannot be recuperated.
As an alternative, we propose a molecular motor (MM) liposome complex that enables light-triggered release through mechanical action without inducing phototoxicity, and allows for controlled step-wise release through reversibility of molecular motion. Most formulations that have been approved in the clinic and are historically much longer investigated, are phospholipid-based structures.4 Previously, light-triggered release using amphiphilic phthalocyanine in conventional liposomes have been designed to release payload using near-infra red28 irradiation but also adding gold to the liposome surface to utilize the plasmon-resonance effect to facilitate release,29 or embedding graphene-oxide inside liposomes as the light-responsive moiety.30 In most cases, light is being transformed into heat that facilitates release from the liposomes but direct interactions have been shown to work as well.31 The alternative molecular motor approach does not rely on heat transfer properties but directly influence the local bilayer organization in a non-destructive fashion.
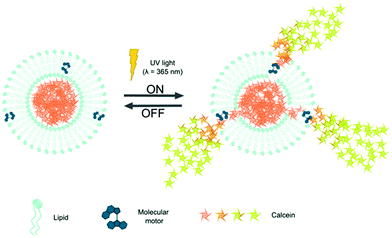
Previously, a tailored amphiphilic molecular motor designed to incorporate into the cell membrane was used to disrupt the lipid bilayers and open up the cell membrane.32 We hypothesized that a relatively simple hydrophobic small unidirectional molecular rotary motor is able to reside inside the hydrophobic domain of the lipid bilayer and open up the membrane of liposomes upon irradiation. Recently, such molecular motor was used to direct stem cell fate.33 These unidirectional rotary motors are tunable in chemistry and rotation speed and offer direct mechanical interaction with their surrounding mediated by light.34 The molecular motor was embedded inside a liposome loaded with calcein to assess on demand release capabilities as depicted in Scheme 1.
The molecular motor (MM) is hydrophobic and similar to other small hydrophobic components can be stored inside the hydrophobic domain of a phospholipid membrane. Upon irradiation with UV-light, the MM undergoes a photoisomerization around the central double bond that is followed by a thermal relaxation step. Two cycles are needed to complete a full rotation (Supp. Info 1, ESI†) and have been shown to function in mixed molecular systems to induce motion.35 Liposomes (single unilamellar vesicles, SUVs) with MM were made at phospholipid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MM weight ratios of 50
MM weight ratios of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (MM1) and 25
1 (MM1) and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
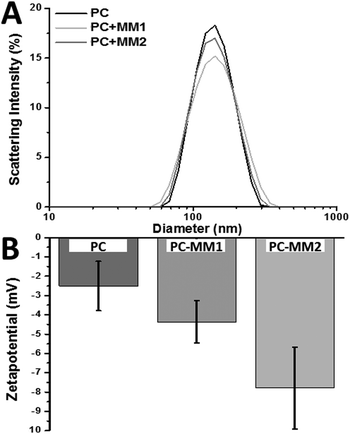
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (MM2), by means of lipid film rehydration and subsequent extrusion (ESI,† materials and methods). Dynamic light scattering analysis, indicated that all liposomes displayed similar sizes with slightly altered distribution (Fig. 1A). However, the zeta-potential of the non-loaded liposomes and MM1 liposomes was similar while MM2 liposomes are more negatively charged (Fig. 1B), indicating that the molecular motor resides more at the surface due to the deprotonated carboxylates and generates a more negative surface.
2 (MM2), by means of lipid film rehydration and subsequent extrusion (ESI,† materials and methods). Dynamic light scattering analysis, indicated that all liposomes displayed similar sizes with slightly altered distribution (Fig. 1A). However, the zeta-potential of the non-loaded liposomes and MM1 liposomes was similar while MM2 liposomes are more negatively charged (Fig. 1B), indicating that the molecular motor resides more at the surface due to the deprotonated carboxylates and generates a more negative surface.
The liposomes were loaded with calcein as a model compound as it is very suitable to analyze calcein release from liposomes based on the self-quenching fluorescent properties of calcein. Liposomes were loaded with a calcein solution at a concentration above the self-quenching concentration, which is maintained inside the liposome. Upon release of calcein into its environment as would occur after destabilization of the loaded liposomes, its concentration will decrease causing an increase in fluorescence. Therefore, released calcein will become clearly measurable and distinguishable from the non-released calcein. By measuring the fluorescence intensity over time and comparing it with full release (complete liposome dissolution using detergents), the release (%) over time was determined.
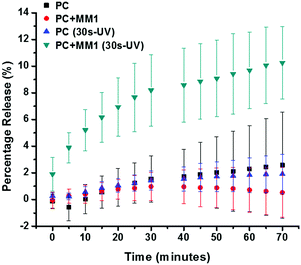
Both control liposomes and MM1 liposomes were subjected to the same treatments and calcein release over time (70 min) was assessed in the absence and presence of irradiation (Fig. 2). MM2 liposomes were not included in the studies as for MM2 without UV-irradiation high leakage was identified (Suppl. Info 2, ESI†) while no significant release was detected for the control liposomes and MM1 liposomes. These results indicate that the low abundance of MM inside the lipid bilayer does not compromise the low permeability of the lipid membrane. Subsequently, calcein-loaded liposomes were irradiated for 30 s at a wavelength of 365 nm. UV irradiation causes the molecular motors to rotate and induce molecular motion.36
With a single irradiation period of 30 s, the irradiated MM1 liposomes displayed enhanced release compared to the control liposomes. A calcein release up to 10% was observed within 60–70 min. after which the release leveled off. The partial release indicates that moderate release is facilitated rather than that all content is liberated at once (burst release), which in turn indicates that the molecular motion of the motor inside the lipid bilayer does not result in destruction of the membrane. Restoration of the membrane integrity allowed the liposome to regain its initial stability. The liposomes without MM that were subjected to UV-irradiation did not display any calcein release, indicating that the liposome integrity was not compromised due to the high energetic irradiation (Fig. 2). Therefore, UV-induced local increase in temperature or molecular alterations to the lipid membrane did not induce the calcein release, but it can be attributed to the rotation of the molecular motor.
The quantified release at intermediate time points further exemplify the difference between MM1 liposomes and control lipsomes (Supp. Info 3, ESI†). It is clearly distinguishable that most of the release occurs shortly after the irradiation-step and only for the liposomes that have the molecular motor incorporated into the lipid membrane. Release of 1% is detected for liposomes without the MM inside the membrane. This result indicates that there is a very minor amount of unspecific release, which was not detected in the samples without irradiation. This release indicates that the UV-irradiation influence the system, most like due to (local) heating that faciliates slight permeabilization of the liposome membrane. However, in none of the cases did the UV-irradiation alter the size of the liposomes (Supp. Info 4, ESI†).
In order to identify the extent of control over the calcein release from MM-containing liposomes, also a higher irradiation time was investigated. By increasing the irradiation dosage, either by time or intensity, the molecular motors would provide enhanced molecular rotatory motion. Liposomes from the same batch prepared with a lipid to MM ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) were subjected to 0, 30, and 60 s of UV-irradiation after which the fluorescence intensity was analyzed over time (Fig. 3). It is clearly visible that upon increasing the irradiation time, the release of calcein is enhanced. After 60 s exposure to UV-light, 18% release was observed compared to about 8% after 30 s exposure. This difference in release profile simply by adjusting the dosage of UV-irradiation illustrates the ease of control over the system. It enables us to tailor the amount of release to meet either the required dosing for an active compound to function, or control the release over several events. The release profile indicates a transient release as about 80% of the maximum release that occurs upon a single irradiation event is obtained within the first 20 minutes after irradiation, and levels off after that. The fact that the release continues for a period beyond the irradiation time, indicates that reestablishing the low permeability of the liposome membrane is a slow process. In conclusion, the calcein release from MM liposomes can be controlled using simple adjustable parameters such as irradiation time and strength, and number of pulses. Unfortunately, irradiation times >60 s and repetitive irradiation inflicted photo-bleaching of calcein, hindering further data analysis on this issue.
1 (w/w) were subjected to 0, 30, and 60 s of UV-irradiation after which the fluorescence intensity was analyzed over time (Fig. 3). It is clearly visible that upon increasing the irradiation time, the release of calcein is enhanced. After 60 s exposure to UV-light, 18% release was observed compared to about 8% after 30 s exposure. This difference in release profile simply by adjusting the dosage of UV-irradiation illustrates the ease of control over the system. It enables us to tailor the amount of release to meet either the required dosing for an active compound to function, or control the release over several events. The release profile indicates a transient release as about 80% of the maximum release that occurs upon a single irradiation event is obtained within the first 20 minutes after irradiation, and levels off after that. The fact that the release continues for a period beyond the irradiation time, indicates that reestablishing the low permeability of the liposome membrane is a slow process. In conclusion, the calcein release from MM liposomes can be controlled using simple adjustable parameters such as irradiation time and strength, and number of pulses. Unfortunately, irradiation times >60 s and repetitive irradiation inflicted photo-bleaching of calcein, hindering further data analysis on this issue.
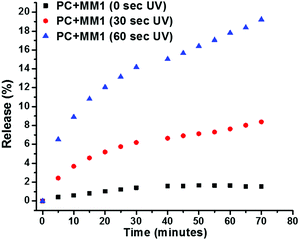 | ||
Fig. 3 Liposomes with MM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) without irradiation and with irradiation for 30 and 60 s of which the calcein release was studied using fluorescence spectroscopy. 50) without irradiation and with irradiation for 30 and 60 s of which the calcein release was studied using fluorescence spectroscopy. | ||
Redesigning new nanocarriers is something that is a challenge but highly important to advance the field of nanomedicine and facilitate controlled, active, on-demand delivery at the right location at the right time. However, while still many new materials are under development and far from clinical application, novel approaches that are more straightforward will provide the opportunity to impact the nanomedicine and controlled release field in an immediate fashion. By bringing together the well-established and clinically relevant liposomal nanocarrier system with the sophisticated responsive small molecular rotating motor, new innovative approaches for controlled release are possible. Although, other photoresponsive structures have been explored such as azobenzene derivatives,37,38 these were inserted into lipid bilayers through the conjugation of an aliphatic tail, thus requiring additional synthesis. Here we show that the UV-induced rotation of a hydrophobic molecular motor, stored inside the lipid membrane, disrupts the membrane to such an extent that small molecules (calcein) are released. This release only occurs in the presence of the molecular motor and combined with UV-irradiation. Without either the molecular motor or the UV-irradiation no significant release was found. An increase in irradiation dose produced enhanced calcein release, which indicates that with this relatively simple approach a high degree of control over drug release can be obtained. The incorporation of such an approach is not limited to phospholipid systems but is also envisioned to be compatible with polymer-based NCs. Recently, both two photon excitation using Near-IR irradiation32 and responsiveness to visible light39 have been explored which will improve the biocompatibility of the NCs.
This work was financially supported by the European Research Council (ERC; advanced grant no. 694345 to B. L. F.), the Dutch Ministry of Education, Culture and Science (Gravitation program no. 024.001.035), the Abel Tasman Talent Program (GSMS-UMCG to L. R.), and the Dutch Technology Foundation TTW, part of The Netherlands Organisation for Scientific Research (NWO), and partly funded by the Ministry of Economic Affairs (I. S. Z.). We thank Eliza Warszawik for helpful discussion. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Conflicts of interest
The authors declare the following competing financial interest(s): P. V. R is co-founder/scientific advisor/shareholder of BiomACS BV. There are no other conflicts to declare.Notes and references
- V. Wagner, A. Dullaart, A. K. Bock and A. Zweck, Nat. Biotechnol., 2006, 24, 1211–1217 CrossRef CAS PubMed.
- Y. Min, J. M. Caster, M. J. Eblan and A. Z. Wang, Chem. Rev., 2015, 115, 11147–11190 CrossRef CAS PubMed.
- R. van der Meel, E. Sulheim, Y. Shi, F. Kiessling, W. J. M. Mulder and T. Lammers, Nat. Nanotechnol., 2019, 14, 1007–1017 CrossRef CAS PubMed.
- Y. Namiki, T. Fuchigami, N. Tada, R. Kawamura, S. Matsunuma, Y. Kitamoto and M. Nakagawa, Acc. Chem. Res., 2011, 44, 1080–1093 CrossRef CAS PubMed.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. D. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, S. Sharma, S. Habtemariam and H. S. Shin, J. Nanobiotechnol., 2018, 16, 1–33 CrossRef PubMed.
- M. B. de Jesus and I. S. Zuhorn, J. Controlled Release, 2015, 201, 1–13 CrossRef CAS PubMed.
- P. R. Cullis and M. J. Hope, Mol. Ther., 2017, 25, 1467–1475 CrossRef CAS PubMed.
- J. R. Ferrer, A. J. Sinegra, D. Ivancic, X. Y. Yeap, L. Qiu, J. J. Wang, Z. J. Zhang, J. A. Wertheim and C. A. Mirkin, ACS Nano, 2020, 14, 1682–1693 CrossRef CAS PubMed.
- G. Hao, Z. P. Xu and L. Li, RSC Adv., 2018, 8, 22182–22192 RSC.
- W. J. Wang, Y. C. Huang, C. M. Su and T. R. Ger, Front. Chem., 2019, 7, 1–11 CrossRef PubMed.
- M. a C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- F. Oroojalian, M. Babaei, S. M. Taghdisi, K. Abnous, M. Ramezani and M. Alibolandi, J. Controlled Release, 2018, 288, 45–61 CrossRef CAS PubMed.
- A. Radaic, E. de Paula and M. De Jesus, J. Nanosci. Nanotechnol., 2014, 14, 1–8 CrossRef PubMed.
- J. A. Kulkarni, D. Witzigmann, S. Chen, P. R. Cullis and R. van der Meel, Acc. Chem. Res., 2019, 52, 2435–2444 CrossRef CAS PubMed.
- Y. Wu, Y. Xiong, L. Wang, Q. Zhou, L. Li, P. A. Levkin, G. Davidson, L. Gao and W. Deng, Biomater. Sci., 2020, 8, 3021–3025 RSC.
- M. R. Molla, A. Böser, A. Rana, K. Schwarz and P. A. Levkin, Bioconjug. Chem., 2018, 29, 992–999 CrossRef CAS PubMed.
- K. O. Affram, T. Smith, E. Ofori, S. Krishnan, P. Underwood, J. G. Trevino and E. Agyare, J. Drug Delivery Sci. Technol., 2020, 55, 101374 CrossRef CAS PubMed.
- B. García-Pinel, C. Porras-Alcalá, A. Ortega-Rodríguez, F. Sarabia, J. Prados, C. Melguizo and J. M. López-Romero, Nanomaterials, 2019, 9, 638 CrossRef PubMed.
- B. Sarmento, S. Martins, D. Ferreira and E. B. Souto, Int. J. Nanomed., 2007, 2, 743–749 CAS.
- E. Andreozzi, J. W. Seo, K. Ferrara and A. Louie, Bioconjugate Chem., 2011, 22, 808–818 CrossRef CAS PubMed.
- A. K. Ayan, A. Yenilmez and H. Eroglu, Mater. Sci. Eng., C, 2017, 75, 663–670 CrossRef CAS PubMed.
- G. A. O. Jinhao, G. U. Hongwei and X. U. Bing, Acc. Chem. Res., 2009, 42(8), 1097–1107 CrossRef PubMed.
- A. Dasgupta, M. Liu, T. Ojha, G. Storm, F. Kiessling and T. Lammers, Drug Discovery Today: Technol., 2016, 20, 41–48 CrossRef.
- L. Beauté, N. McClenaghan and S. Lecommandoux, Adv. Drug Delivery Rev., 2019, 138, 148–166 CrossRef PubMed.
- Z. Sun, G. Liu, J. Hu and S. Liu, Biomacromolecules, 2018, 19, 2071–2081 CrossRef CAS PubMed.
- Y. Duan, Y. Wang, X. Li, G. Zhang, G. Zhang and J. Hu, Chem. Sci., 2019, 11, 186–194 RSC.
- G. Ioele, M. De Luca, A. Garofalo and G. Ragno, Drug Delivery, 2017, 24, 33–44 CrossRef CAS PubMed.
- M. Macháček, K. A. Carter, F. Kostelanský, D. Miranda, A. Seffouh, J. Ortega, T. Šimůnek, P. Zimčík and J. F. Lovell, J. Mater. Chem. B, 2018, 6, 7298–7305 RSC.
- T. S. Troutman, S. J. Leung and M. Romanowski, Adv. Mater., 2009, 21, 2334–2338 CrossRef CAS PubMed.
- A. Sahu, M. Kim, J. Ryu, J. G. Son, E. Lee, D. Y. Noh and G. Tae, Mater. Sci. Eng., C, 2018, 82, 19–24 CrossRef CAS PubMed.
- A. Koçer, M. Walko, W. Meijberg and B. L. Feringa, Science, 2005, 309, 755–758 CrossRef PubMed.
- D. Liu, V. Garcia-Lopez, R. S. Gunasekera, L. Greer Nilewski, L. B. Alemany, A. Aliyan, T. Jin, G. Wang, J. M. Tour and R. Pal, ACS Nano, 2019, 13, 6813–6823 CrossRef CAS PubMed.
- Q. Zhou, J. Chen, Y. Luan, P. A. Vainikka, S. Thallmair, S. J. Marrink, B. L. Feringa and P. van Rijn, Sci. Adv., 2020, 6, eaay2756 CrossRef PubMed.
- J. Chen, K. Chen, G. T. Carroll and B. L. Feringa, Chem. Commun., 2014, 50, 12641–12644 RSC.
- J. Vicario, N. Katsonis, B. Serrano Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 163 CrossRef PubMed.
- J. Chen, K.-Y. Chen, G. T. Carroll and B. L. Feringa, Chem. Commun., 2014, 50, 12641–12644 RSC.
- P. Urban, S. D. Pritzl, M. F. Ober, C. F. Dirscherl, C. Pernpeintner, D. B. Konrad, J. A. Frank, D. Trauner, B. Nickel and T. Lohmueller, Langmuir, 2020, 36, 2629–2634 CrossRef CAS PubMed.
- J. M. Kuiper and J. B. F. N. Engberts, Langmuir, 2004, 20, 1152–1160 CrossRef CAS PubMed.
- L. Pfeifer, M. Scherübl, M. Fellert, W. Danowski, J. Cheng, J. Pol and B. L. Feringa, Chem. Sci., 2019, 10, 8768–8773 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Method section, zeta-potential measurements, and quantified release percentage of fluorescent dye. See DOI: 10.1039/d0cc02499f |
| This journal is © The Royal Society of Chemistry 2020 |