 Open Access Article
Open Access ArticleDissolution of noble metals in highly concentrated acidic salt solutions†
Federica
Forte
 ,
Sofia
Riaño
,
Sofia
Riaño
 and
Koen
Binnemans
and
Koen
Binnemans
 *
*
KU Leuven, Department of Chemistry, Celestijnenlaan 200F, PO box 2404, 3001 Leuven (Heverlee), Belgium. E-mail: Koen.Binnemans@kuleuven.be
First published on 19th June 2020
Abstract
Highly concentrated solutions of AlCl3·6H2O and Al(NO3)3·9H2O were investigated for Au and platinum group metals dissolution. 95% Pd was leached from spent automotive catalysts in 15 min at 80 °C, while Pt required longer times; Rh dissolution was <20%. Au dissolution from wires required 24 h. Pd recovery was investigated by reductive precipitation.
Strongly oxidizing conditions are required for the dissolution of noble metals. Aqua regia, a mixture of concentrated nitric acid and concentrated hydrochloric acid in a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 volume ratio, is a well-known lixiviant for gold, platinum and palladium, while rhodium can be dissolved if it is in a finely divided form. Several recycling processes to recover precious metals from electronic scrap and spent catalysts make use of aqua regia.1–4 Although aqua regia is still often used, both in research laboratories, in industry and by jewelers, it is a potentially dangerous solvent mixture and there are environmental concerns related to its use.5
3 volume ratio, is a well-known lixiviant for gold, platinum and palladium, while rhodium can be dissolved if it is in a finely divided form. Several recycling processes to recover precious metals from electronic scrap and spent catalysts make use of aqua regia.1–4 Although aqua regia is still often used, both in research laboratories, in industry and by jewelers, it is a potentially dangerous solvent mixture and there are environmental concerns related to its use.5
Many researchers have been trying to find alternatives for aqua regia as a solvent for noble metals, including platinum-group metals (PGMs). For instance, “organic aqua regia (OAR)”, which is composed of thionyl chloride (SOCl2) and a polar aprotic organic solvent such as pyridine, imidazole or N,N-dimethylformamide (DMF), has been proposed as a safer alternative for aqua regia.6–8 Its composition can be tuned to selectively dissolve gold or palladium, but not platinum. However, SOCl2 cannot be considered as an environmentally friendly compound, neither are the polar aprotic solvents. Many studies have been devoted to the dissolution of gold in organic solvent mixtures.9–13 Recently, polyhalide ionic liquids have been found to be powerful solvents for oxidative dissolution of noble metals.14,15 FeCl3–KCl mixtures have been presented as “dry aqua regia”.16 Angelidis and Skouraki proposed the use of aluminum chloride solutions, with low concentrations of nitric acid as an oxidant, instead of aqua regia, for the recovery of platinum from spent automotive catalysts.17
The work reported here was inspired by a paper by Angell and coworkers from 1973 in which it was remarked that concentrated solutions of AlCl3·6H2O and Al(NO3)3·9H2O dissolve noble metals at a much greater rate than can be achieved with boiling aqua regia.18 Although the authors wrote that this phenomenon would be described in a follow-up paper, it has never been published.19 Concentrated solutions of salts of highly valent metal ions, having an H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal-ion ratio sufficiently low to minimize outer-sphere hydration, show a marked degree of acidity which can be exploited for the dissolution of noble metals.20 Such highly concentrated salt solutions have a limited amount of free water molecules and are closely related to molten salt hydrates and deep-eutectic solvents based on hydrated metal salts.21–23
metal-ion ratio sufficiently low to minimize outer-sphere hydration, show a marked degree of acidity which can be exploited for the dissolution of noble metals.20 Such highly concentrated salt solutions have a limited amount of free water molecules and are closely related to molten salt hydrates and deep-eutectic solvents based on hydrated metal salts.21–23
In this communication, a very concentrated mixture of hydrated AlCl3 and Al(NO3)3 (75–85 wt% hydrated salts and 15–25 wt% added water) was used as leaching agent. It was tested on both metal wires and on a real matrix, comprising spent automotive catalysts. The recovery of PGMs from the loaded leachate was investigated by reductive precipitation with ascorbic acid, which is an effective agent for PGMs recovery from both chloride and nitrate media.24,25
Preliminary leaching tests were performed on palladium wires by using the following conditions: salt/wire (S/W) ratio = 35 g/g, T = 80 °C, stirring speed = 350 rpm. Equal amounts of hydrated AlCl3 and Al(NO3)3 were used in each experiment. The extra water added to the system was 22 wt%; this value represented the percentage of the water added to the system (g) with respect to the total mass of the system (g), as reported in eqn (1):
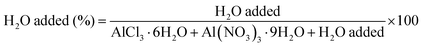 | (1) |
Already after 3 min, the wire started to dissolve. After 1 h, the whole leachate turned reddish (Fig. S1a, ESI†). Full dissolution was obtained after 4.5 h (Fig. S1f, ESI†). The same conditions (T = 80 °C, S/W = 35 g/g, 22 wt% water added, stirring speed = 350 rpm) were applied for the dissolution of gold wire (Fig. S2, ESI†). In this case, full dissolution was observed only after 24 h. This could be explained with the higher thickness of the gold wire (1 mm) compared to the palladium wire (0.5 mm). Extra tests were performed on platinum and rhodium wires. It was found that Pt was fully dissolved after a couple of days whereas the Rh wire could not be dissolved.
The effectiveness of the leaching system was also tested on a real matrix comprising spent automotive catalysts with the following PGMs content: [Pd] = 2867 ppm, [Pt] = 1533 ppm, [Rh] = 835 ppm. In Fig. 1, the leaching efficiency is reported as a function of the salt-to-catalyst (S/C) ratio. 35 g/g was found to be the optimal ratio at the tested experimental conditions (T = 80 °C, t = 1 h, water added = 26 wt%, stirring speed = 350 rpm) in terms of leaching efficiency, since it gave the highest yield for palladium dissolution. These conditions were then applied in the next series of leaching experiments, where the influence of the contact time was investigated (Fig. 2). It was found that full dissolution of all PGMs (Pd, Pt, Rh) could not be achieved easily, but a selectivity for palladium was observed by using short contact times (lower than 30 min). The highest platinum dissolution (about 64%) was obtained at t = 4 h; a further increase of the contact time did not bring additional platinum or rhodium in solution.
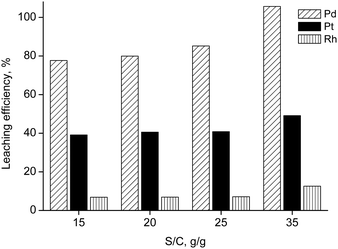 | ||
| Fig. 1 Leaching as a function of the salt-to-catalyst (S/C) ratio (T = 80 °C, t = 1 h, water added = 26 wt%, stirring speed = 350 rpm). | ||
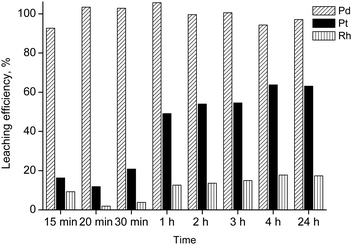 | ||
| Fig. 2 Leaching as a function of time (salt-to-catalyst ratio = 35 g/g, T = 80 °C, water added = 26 wt%, stirring speed = 350 rpm). | ||
Another set of experiments was performed to investigate the influence of the water content (Table 1). R is here defined, according to Sare et al., as shown in eqn (2).18
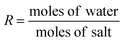 | (2) |
| Water added, wt% | R (Nwater/Nsalt) | Leaching efficiency (%) | ||
|---|---|---|---|---|
| Pd | Pt | Rh | ||
| 14 | 9.7 | 94 | 51 | 12 |
| 17 | 10.4 | 96 | 54 | 14 |
| 20 | 11.4 | 91 | 48 | 20 |
| 22 | 11.8 | 97 | 51 | 13 |
| 26 | 13.0 | 94 | 64 | 18 |
The moles of water refer to the water already present in the salts plus the water added. It was found that 90–95% palladium dissolution could be achieved in the tested experimental conditions. A decrease of the water content has an effect mostly on platinum leaching, which decreased from 64 to 51%; this result can be explained by the improved mass transfer properties of the system when using a higher water content. Tests with water content higher than 26 wt% were not performed to avoid strong dilution of the system without having a significant increase in palladium leaching.
The leaching mechanism can be explained with the oxidation of metals by HNO3; the oxidized ions then react with chloride ions resulting in hexachloroplatinate(IV) (PtCl62−) ions, as shown in eqn (3) and (4):
| Pt(s) + 4NO−3(aq) + 8H+ → Pt4+(aq) + 4NO2(g) + 4H2O(aq) | (3) |
| Pt4+(aq) + 6Cl−(aq) + 8H+ → PtCl2−6(aq) | (4) |
Leaching requires an oxidising agent such as HNO3. Its reduction potential is high enough to allow the oxidation reaction to take place.26 The leaching mechanism is mainly based on the dissolution of Pt and the formation of chloro complexes that are quite stable at pH < 1, while at higher pH the complexes may hydrolyse forming hydroxy complexes.27
From these tests, it is evident that leaching of palladium was the easiest one, while leaching of platinum and rhodium was more difficult. This result might be explained with the higher electrochemical potential of Pd and with the higher stability of chloropalladate(II) complexes at low pH. A possible approach for PGMs recovery from the catalyst might include a first leaching step at t = 15 min in order to obtain quantitative dissolution of palladium. The residue, rich in platinum and rhodium, could be further treated through a second leaching step by employing longer contact times (4 h) to dissolve about 65% platinum. A multi-stage leaching process, where fresh solvent is used each time, would help to improve the dissolution of Pt and Rh.
A leaching step on a larger scale (1 g of catalyst powder) was finally performed using the following conditions: salt-to-catalyst ratio = 35 g/g, water added = 26 wt%, t = 15 min, stirring speed = 350 rpm, in order to get a leachate enriched in palladium for further recovery. This leachate was also analysed for other elements, such as aluminium, cerium, iron and zinc (Table 2). As expected, the leachate was strongly contaminated by aluminium. It is therefore crucial to identify a proper treatment process to recover the PGMs in a selective way, minimizing Al contamination.
| Metal | Concentration, ppm |
|---|---|
| Al | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 259 259 |
| Ce | 66 |
| Fe | 30 |
| Zn | 40 |
| Pd | 55 |
| Pt | 3 |
| Rh | 1 |
The recovery of palladium was investigated by reductive precipitation with ascorbic acid. First, precipitation tests were performed on a leachate obtained from the dissolution of palladium wire. Equal volumes of the leachate and a solution of ascorbic acid 1 mol L−1 in water were contacted. Samples were shaken for 5 min and afterwards allowed to settle. At first, no changes to the solution were observed, but after three days, a metallic precipitate was found on the bottom of the vial (Fig. S3, ESI†). In order to determine its composition, the precipitate was washed with water, dried (T = 105 °C, t = 2 h) and re-dissolved in 65% HNO3 for ICP analysis. As expected from the reductive potentials of palladium and aluminium, only palladium was precipitated from the leachate, while aluminium remained in solution. Standard reduction potentials (E°/V) are −1.662 for Al/Al3+ and +0.951 for Pd/Pd2+.28 The amount of precipitated palladium was about 25%, so further investigation is required to fully optimize this step, for example by testing higher temperature values. The palladium precipitation reaction can be express as:
| Pd2+ + H2A ↔ Pd(s) + A + 2H+ | (5) |
The precipitation tests were also performed on the leachate originating from the dissolution of the catalysts. After the leaching, the solid residue (salt and catalyst not dissolved) was separated from the leachate by centrifugation. The leachate was put in contact with an equal volume of an ascorbic acid solution in water. Three different concentrations were tested (0.1 mol L−1, 0.5 mol L−1 and 1 mol L−1). Once more, no precipitation of aluminium was observed and palladium precipitation was 25% for all the tested solutions. Both platinum and rhodium quantitatively precipitated; however, considering that the leaching of Pd at the optimised conditions showed a good selectivity for Pd (see Table 2), the purity of the obtained precipitate is relatively high (77% purity; calculated value based on Al, Pd, Pt and Rh analysis).
In conclusion, highly concentrated solutions comprising mixtures of hydrated aluminium nitrate and chloride salts were tested for the dissolution of PGMs. The leaching system was tested both on metal wires and on real material (spent automotive catalysts). Quantitative dissolution of palladium from the catalysts powder was achieved in a short time (15 min); about 64% platinum can be dissolved when the contact time is increased up to 4 h. Further investigation is required in order to optimize PGMs recovery via reductive precipitation with ascorbic acid.
This work has received funding from the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 730224 (PLATIRUS: PLATInum group metals Recovery Using Secondary raw materials) (project website: http://www.platirus.eu/). The authors acknowledge Monolithos (Athens, Greece) for providing the catalysts.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. J. Park and D. J. Fray, J. Hazard. Mater., 2009, 164, 1152–1158 CrossRef CAS PubMed.
- A. G. Chmielewski, T. S. Urbanski and W. Migdal, Hydrometallurgy, 1997, 45, 333–344 CrossRef CAS.
- P. P. Sheng and T. H. Etsell, Waste Manage. Res., 2007, 25(4), 380–383 CrossRef CAS PubMed.
- M. Baghalha, Gh. H. Khosravian and H. R. Mortaheb, Hydrometallurgy, 2009, 95, 247–253 CrossRef CAS.
- M. Gökelma, A. Birich, S. Stopic and B. Friedrich, J. Mater. Sci. Chem. Eng., 2016, 4, 8–17 Search PubMed.
- W. Lin, R. W. Zhang, S. S. Jang, C. P. Wong and J. Hong, Angew. Chem., Int. Ed., 2010, 49, 7929–7932 CrossRef CAS PubMed.
- W. Lin, Rare Met., 2012, 31, 92–95 CrossRef CAS.
- J. Zhao, B. Wang, X. Xu, Y. Yu, S. Di, H. Xu, Y. Zhai, H. He, L. Guo, Z. Pan and X. Li, J. Catal., 2017, 350, 149–158 CrossRef CAS.
- K. Binnemans and P. T. Jones, J. Sustain. Metall., 2017, 3, 570–600 CrossRef.
- A. Serpe, F. Artizzu, M. L. Mercuri, L. Pilia and P. Deplano, Coord. Chem. Rev., 2008, 252, 1200–1212 CrossRef CAS.
- M. Räisänen, E. Heliövaara, F. Al-Qaisi, M. Muuronen, A. Eronen, H. Liljeqvist, M. Nieger, M. Kemell, K. Moslova, J. Hämäläinen, K. Lagerblom and T. Repo, Angew. Chem., Int. Ed., 2018, 57, 17104–17109 CrossRef PubMed.
- Y. Nakao and K. Sone, Chem. Commun., 1996, 897–898 RSC.
- J. J. M. Nelson and E. J. Schelter, Inorg. Chem., 2019, 58, 979–990 CrossRef CAS PubMed.
- X. Li, A. Van den Bossche, T. Vander Hoogerstraete and K. Binnemans, Chem. Commun., 2018, 54, 475–478 RSC.
- A. Van den Bossche, E. De Witte, W. Dehaen and K. Binnemans, Green Chem., 2018, 20, 3327–3338 RSC.
- A. Yoshimura and Y. Matsuno, J. Jpn. Inst. Met., 2019, 83, 23–29 CrossRef CAS.
- T. N. Angelidis and S. Skouraki, Appl. Catal., A, 1996, 142, 387–395 CrossRef CAS.
- E. J. Sare, C. T. Moynihan and C. A. Angell, J. Phys. Chem., 1973, 77, 1869–1876 CrossRef CAS.
- C. A. Angell, N. Byrne and J. P. Belieres, Acc. Chem. Res., 2007, 40, 1228–1236 CrossRef CAS PubMed.
- J. A. Duffy, Inorg. Chem., 1977, 16, 2988 CrossRef CAS.
- H. H. Emons, Electrochim. Acta, 1988, 33, 1243–1250 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies and R. K. Rasheed, Chem. – Eur. J., 2004, 10, 3769–3774 CrossRef CAS PubMed.
- E. L. Smith, A. P. Abbott, P. Andrew and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- S. H. Lee, K. R. Kim, J. S. Shon, J. H. Yoo and H. Chung, Korean J. Chem. Eng., 1999, 16, 166–169 CrossRef CAS.
- T. Phetla, E. Muzenda and M. Belaid, Int. J. Chem. Eng., 2010, 4, 573–579 Search PubMed.
- L. Pietrelli and D. Fontana, Int. J. Environ. Waste Manage., 2013, 11, 222–232 CrossRef CAS.
- T. N. Angelidis and E. Skouraki, Appl. Catal., A, 1996, 142, 387–395 CrossRef CAS.
- P. Vanýsek, Electrochemical Series. CRC Handbook of Chemistry and Physics, Taylor & Francis Inc, Boca Raton, Florida, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: List of chemicals, analytical techniques, characterisation, leaching, precipitation. See DOI: 10.1039/d0cc02298e |
| This journal is © The Royal Society of Chemistry 2020 |
