Chirality induction on non-chiral dye-linked polysilsesquioxane in nanohelical structures†
Abstract
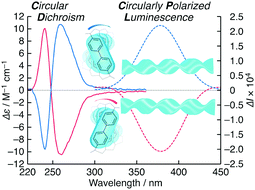
We demonstrate the direct induction of chirally arranged organic dye-linked polysilsesquioxane through a sol–gel transcription using a chiral supramolecular template. The chiral arrangement was confirmed by using electronic and vibrational circular dichroism and circularly polarized luminescence spectroscopies.


 Please wait while we load your content...
Please wait while we load your content...