Enantioselective formal synthesis of the marine macrolide (−)-callyspongiolide†
Abstract
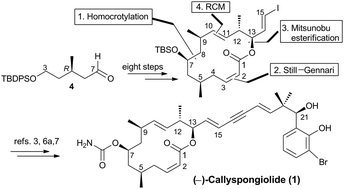
A short enantioselective synthesis of the macrocyclic core 19 of callyspongiolide, involving a homocrotylboration of aldehyde 4, a Still–Genari olefination, an esterification with alcohol 17, and a ring-closing metathesis, is reported.


 Please wait while we load your content...
Please wait while we load your content...