Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boronate ester cross-linked PVA hydrogels for the capture and H2O2-mediated release of active fluorophores†
George T.
Williams‡
 ab,
Adam C.
Sedgwick‡
ab,
Adam C.
Sedgwick‡
 c,
Sajal
Sen‡
c,
Sajal
Sen‡
 c,
Lauren
Gwynne
c,
Lauren
Gwynne
 a,
Jordan E.
Gardiner
a,
James T.
Brewster
II
a,
Jordan E.
Gardiner
a,
James T.
Brewster
II
 c,
Jennifer R.
Hiscock
c,
Jennifer R.
Hiscock
 *b,
Tony D.
James
*b,
Tony D.
James
 *a,
A. Toby A.
Jenkins
*a,
A. Toby A.
Jenkins
 *a and
Jonathan L.
Sessler
*a and
Jonathan L.
Sessler
 *c
*c
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; a.t.a.jenkins@bath.ac.uk
bSchool of Physical Sciences, University of Kent, Canterbury, CT2 7NH, UK. E-mail: J.R.Hiscock@kent.ac.uk
cDepartment of Chemistry, University of Texas at Austin, 105 E 24th street A5300, Austin, TX 78712-1224, USA. E-mail: sessler@cm.utexas.edu
First published on 7th April 2020
Abstract
A new set of PVA hydrogels were formed using the boronate ester fluorescent probe PF1 and the novel boronate fluorescent probe PT1 as the covalent crosslinkers. Treatment with aqueous H2O2 allowed triggered release of the fluorescent dye accompanied by complete dissolution of the hydrogel.
Functional hydrogels have generated widespread interest as so-called intelligent devices wherein a specific stimulus can yield a macroscopic change to the self-supporting material.1,2 Such constructs offer promise in the area of drug delivery and design of “smart” wound dressings.3–6 In addition, these functional hydrogels have demonstrated great potential as fluorescent probes for live cell imaging, disease diagnosis and sensing applications with the controlled release of a fluorophore.7 These constructs have utilised non-covalent interactions such as aromatic–aromatic, hydrogen bonding, and hydrophobic interactions. Unfortunately, these interactions can result in the unwanted leaching of the active molecule from the hydrogel matrix. Next generation systems comprised of a pro-molecule backbone covalently linked to the hydrogel may address these issues by providing a higher local dose and sustained/controlled release of the bioactive molecule.8 Here, we demonstrate a new set of controlled release materials wherein hydrogen peroxide (H2O2) is used as a stimulus to release fluorophores from polyvinyl alcohol (PVA) boronate hydrogels.
Boronic acid and boronate esters have found widespread application in material-based applications, in part because of their propensity to bind reversibly with 1,2-and 1,3-diols.9–19
Such chemistry has been demonstrated inter alia using commercially available PVA and diboronic acid crosslinkers to afford functional PVA–boronate hydrogels.20–25 Boronic acids and boronate esters are well-known to undergo H2O2-mediated oxidative transformations to afford their corresponding phenol functionalites.26–28 We envisaged that the use of bis-boronate-based pro-molecules as cross-linkers would afford a H2O2-responsive hydrogel platform that would allow the controlled and localised release of an active molecule, such as a fluorophore (Scheme 1). It is important to note the boronate functionality is commonly used to mask active therapeutics.29,30 Currently, there is considerable interest in functionalized hydrogels wherein a specific stimulus can yield a macroscopic change to the self-supporting material, including for the stimulus-based release of specific payloads.31–34 However, new approaches to achieving such overarching objectives are still needed.
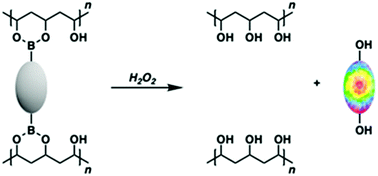 | ||
| Scheme 1 Cartoon representation illustrating the boronate pro-fluorophore encapsulated within a PVA hydrogel being activated by H2O2 to release the active fluorophore. | ||
To address the above need, we have now prepared a new class of H2O2-responsive PVA–boronate hydrogels. These systems rely on covalent cross-linking provided solely by a set of constituent H2O2-responsive boronate ester fluorescent probes, namely the known fluorophore PF126 and the novel fluorescent probe, PT1 (Fig. 1). The resultant hydrogel constructs Greenment (Gment) and Purplement (Pment) displayed stability over 7 days in both aqueous solution and in the air; however, upon exposure to aqueous H2O2 the polymers were oxidised thus releasing their constituent fluorophores, fluorescein26 and thionol35 (Schemes S1 and S2, ESI†). Complete dissolution of the hydrogel could be effected depending on the specific choice of conditions as detailed below.
PF1 was prepared following literature procedures.26 The novel fluorescent probe PT1 was synthesized through the dibromination of commercially available phenothiazine (1) using Br2 (5 equiv.) in acetic acid at room temperature, giving the desired product in 74% yield. Subsequent Suzuki–Miyaura borylation using potassium acetate, bis(pinacolato)diboron, and Pd(dppf)Cl2 afforded PT1 in 43% yield.
With PF1 and PT1 in hand, UV and fluorescence analyses were performed. Upon exposure to aqueous H2O2 at concentrations as low as 125 μM, PF1 exhibited a colour change from clear to green with an increase in absorption at 490 nm and an increase in fluorescence emission at 520 nm, which corresponded to the release of fluorescein (Fig. S1 and S2, ESI†). Whereas, exposure of PT1 to H2O2 in an analogous manner led to a colour change from clear to purple and a concomitant increase in the absorption intensity at 595 nm and an increase in fluorescence emission at 610 nm. These optical changes reflected the release of free thionol as confirmed by high resolution mass spectrometry, Fig. S3–S5 (ESI†). It is important to note that in this work, we have focused on the use of these boronate-based hydrogels as materials whose controlled release may be triggered by H2O2. However, previous reports have demonstrated the greater reactivity of boronate-based fluorescent probes towards peroxynitrite (ONOO−).16,36–39 Therefore, it is likely that if used in cellular applications, both PF1 and PT1 could have a role to play in the fluorescence imaging of both ONOO− and H2O2, albeit not necessarily in a species specific manner.
Next, the Gment and Pment PVA-hydrogels were prepared by mixing a solution of either PF1 or PT1 (100 mM) in dimethylsulfoxide (DMSO) with a DMSO solution of 10% PVA (low molecular weight; purchased commercially) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. This solution was then heated to induce gelation, followed by heating at 60 °C overnight in an oven. The resultant gels were washed with hexanes to remove the displaced pinacol and water to remove excess DMSO. These self-supporting gels proved physically robust and stable in air and could be stored in aqueous media (PBS, pH 7.4) without degradation for 7 days until used Fig. S6–S8 (ESI†).
1 ratio. This solution was then heated to induce gelation, followed by heating at 60 °C overnight in an oven. The resultant gels were washed with hexanes to remove the displaced pinacol and water to remove excess DMSO. These self-supporting gels proved physically robust and stable in air and could be stored in aqueous media (PBS, pH 7.4) without degradation for 7 days until used Fig. S6–S8 (ESI†).
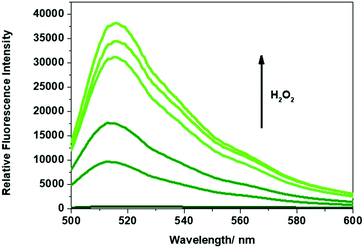
The ability of Gment or Pment-based PVA-hydrogel to release the corresponding dye in the presence of H2O2 was then evaluated. This was done by submerging the chosen hydrogel (200 ± 10 mg) in aqueous solutions containing different concentrations of H2O2. As shown in Fig. 2, exposure of Gment gels to H2O2 (0–1 mM) led to a dose-dependent increase in the fluorescence emission intensity. The colorimetric nature of Gment was then tested by placing the hydrogel (200 ± 10 mg samples) in an aqueous solution of H2O2 (1 mL, 1 mM). A change in colour from colourless to green ensued. Analysis of the UV-Vis absorption revealed an increase in two absorption peaks at 450 nm and 490 nm (Fig. S9, ESI†). The absorption peak at ∼450 nm is tentatively assigned to the release of monoboronate PF340, while the absorption peak at 490 nm corresponds to the release of fluorescein. Based on this result, we believe that oxidation of only one boronate linkage is required to release the fluorescent cargo from the PVA-hydrogel system (Scheme S3, ESI†).
As shown in Fig. 3, Pment PVA-hydrogels exposed to various concentrations of H2O2 (0–1 mM) also led to a dose-dependent increase in the fluorescence intensity at the emission maximum of 610 nm. The colorimetric nature of Pment was then tested by placing the hydrogel (200 ± 10 mg samples) in an aqueous solution of H2O2 (1 mL, 1 mM). A readily discernible change in colour was observed from colourless to purple with an increase in the absorption intensity at 595 nm (see ESI† – Fig. S9–S11). In comparison to one another, Gment was found to be more sensitive to H2O2 than Pment (Fig. S12 and S13, ESI†). This finding is reflected in Gment having a lower Limit of Detection ((LoD) – Gment = 0.12 mM, LoD Pment = 0.33 mM). However, it is important to note, these calculated LoD values are dependent upon incubation times.
Notably, subjecting the hydrogels to an aqueous solution of H2O2 (100 mM) resulted in the complete dissolution of the hydrogels into solution, as shown in Fig. 4. Of note is that commercially available 3% H2O2 sold for consumer use is approximately 980 mM. The present work thus demonstrates the potential utility of boronate-based PVA polymers as a smart material for the masking and facile release of easy-to-visualise fluorophores using a readily accessible trigger. Lastly, an MTT assay with A549 cells was carried out using the Gment gel. At concentrations up to 50 μg mL−1 (note – PVA mw 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 − 23
000 − 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa), A549 cells displayed at least 80% viability, thus demonstrating minimal acute cytotoxicity in this well-studied cell line (Fig. S14, ESI†). We believe these findings provide further support for the suggestion that the present approach may prove useful in achieving the controlled delivery of fluorescence-based diagnostics and active pharmacophores.29
000 kDa), A549 cells displayed at least 80% viability, thus demonstrating minimal acute cytotoxicity in this well-studied cell line (Fig. S14, ESI†). We believe these findings provide further support for the suggestion that the present approach may prove useful in achieving the controlled delivery of fluorescence-based diagnostics and active pharmacophores.29
 | ||
| Fig. 4 (a) Gment and (b) Pment PVA-hydrogels before and after 1.5 h exposure to 100 mM H2O2 in PBS, pH 7.4. | ||
In conclusion, we report here the synthesis of a new H2O2-responsive bis-boronate fluorescent probe, PT1, and the synthesis of the previously reported H2O2-responsive fluorescent probe PF1. Both PT1 and PF1 were successfully used as diboronic acid crosslinkers to form air and aqueous stable PVA-based hydrogels. Exposure of these initially colourless and non-fluorescent systems to aqueous solutions of H2O2 allowed for the controlled release and activation of the encapsulated fluorophore. We believe these systems serve to illustrate a masking and delivery strategy that has the potential to achieve the controlled and localised release of boronic acid-based sensors and pro-drugs.29
The authors would like to thank the EPSRC for grant EP/R003556/1. G. T. W. would like to thank the EPSRC and Public Health England. G. T. W. and J. R. H. would like to thank the global challenges doctoral centre at the University of Kent for funding. A. C. S. and A. T. A. J. wish to thank the EPSRC for funding on smart-wound plasma – EP/R003939/1. This work was supported in part by grant MR/N0137941/1 for the GW4 BIOMED DTP, awarded to the Universities of Bath, Bristol, Cardiff and Exeter from the Medical Research Council (MRC)/UKRI. T. D. J. wishes to thank the Royal Society for a Wolfson Research Merit Award. The work in Austin was supported by the National Institutes of Health (R01 GM103790 to J. L. S.) and the Robert A. Welch Foundation (F-0018 to J. L. S.). A. C. S. would also like to acknowledge use of a Bruker AVIII HD 500 with Prodigy liquid nitrogen cryoprobe supported by NIH grant 1 S10 OD021508. All data supporting this study are provided as ESI† accompanying this paper.
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. N. Ferreira, L. M. B. Ferreira, V. M. O. Cardoso, F. I. Boni, A. L. R. Souza and M. P. D. Gremiao, Eur. Polym. J., 2018, 99, 117–133 CrossRef CAS.
- K. Wang, Y. T. Hao, Y. N. Wang, J. Y. Chen, L. Z. Mao, Y. D. Deng, J. L. Chen, S. J. Yuan, T. T. Zhang, J. Y. Ren and W. Z. Liao, Int. J. Polym. Sci., 2019 DOI:10.1155/2019/3160732.
- M. M. S. Ebrahimi, M. Laabei, A. T. A. Jenkins and H. Schonherr, Macromol. Rapid Commun., 2015, 36, 2123–2128 CrossRef CAS PubMed.
- N. T. Thet, D. R. Alves, J. E. Bean, S. Booth, J. Nzakizwanayo, A. E. R. Young, B. V. Jones and A. T. A. Jenkins, ACS Appl. Mater. Interfaces, 2016, 8, 14909–14919 CrossRef CAS PubMed.
- D. Huber, G. Tegl, A. Mensah, B. Beer, M. Baumann, N. Borth, C. Sygmund, R. Ludwig and G. M. Guebitz, ACS Appl. Mater. Interfaces, 2017, 9, 15307–15316 CrossRef CAS PubMed.
- H. Liu, C. Y. Wang, C. Li, Y. G. Qin, Z. H. Wang, F. Yang, Z. H. Li and J. C. Wang, RSC Adv., 2018, 8, 7533–7549 RSC.
- N. Mehwish, X. Q. Dou, Y. Zhao and C. L. Feng, Mater. Horiz., 2019, 6, 14–44 RSC.
- S. Akkad and C. J. Serpell, Macromol. Rapid Commun., 2018, 39 DOI:10.1002/marc.201800182.
- H. Matsumura, N. Ahmatjan, Y. Ida, R. Imai and K. Wanatabe, Int. Wound J., 2013, 10, 291–294 CrossRef PubMed.
- A. Matsumoto and Y. Miyahara, Sci. Technol. Adv. Mater., 2017, 19, 18–30 CrossRef PubMed.
- A. Matsumoto, K. Kataoka and Y. Miyahara, Polym. J., 2014, 46, 483–491 CrossRef CAS.
- M. Sanjoh, Y. Miyahara, K. Kataoka and A. Matsumoto, Anal. Sci., 2014, 30, 111–117 CrossRef CAS PubMed.
- A. Matsumoto, M. Tanaka, H. Matsumoto, K. Ochi, Y. Moro-oka, H. Kuwata, H. Yamada, I. Shirakawa, T. Miyazawa, H. Ishii, K. Kataoka, Y. Ogawa, Y. Miyahara and T. Suganami, Sci. Adv., 2017, 3 DOI:10.1126/sciadv.aaq0723.
- A. Matsumoto, K. Yamamoto, R. Yoshida, K. Kataoka, T. Aoyagi and Y. Miyahara, Chem. Commun., 2010, 46, 2203–2205 RSC.
- W. L. A. Brooks and B. S. Sumerlin, Chem. Rev., 2016, 116, 1375–1397 CrossRef CAS PubMed.
- M. L. Odyniec, A. C. Sedgwick, A. H. Swan, M. Weber, T. M. S. Tang, J. E. Gardiner, M. Zhang, Y. B. Jiang, G. Kociok-Kohn, R. B. P. Elmes, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2018, 54, 8466–8469 RSC.
- E. V. Lampard, A. C. Sedgwick, T. Sombuttan, G. T. Williams, B. Wannalerse, A. T. A. Jenkins, S. D. Bull and T. D. James, ChemistryOpen, 2018, 7, 266–268 CrossRef CAS PubMed.
- X. L. Sun, M. L. Odyniec, A. C. Sedgwick, K. Lacina, S. Y. Xu, T. T. Qiang, S. D. Bull, F. Marken and T. D. James, Org. Chem. Front., 2017, 4, 1058–1062 RSC.
- B. L. Patenall, G. T. Williams, L. Gwynne, L. J. Stephens, E. V. Lampard, H. J. Hathaway, N. T. Thet, A. E. Young, M. J. Sutton, R. D. Short, S. D. Bull, T. D. James, A. C. Sedgwick and A. T. A. Jenkins, Chem. Commun., 2019, 55, 15129–15132 RSC.
- G. M. Peters, X. D. Chi, C. Brockman and J. L. Sessler, Chem. Commun., 2018, 54, 5407–5409 RSC.
- R. Nishiyabu, S. Ushikubo, Y. Kamiya and Y. Kubo, J. Mater. Chem. A, 2014, 2, 15846–15852 RSC.
- R. Nishiyabu, Y. Takahashi, T. Yabuki, S. Gommori, Y. Yamamoto, H. Kitagishi and Y. Kubo, RSC Adv., 2020, 10, 86–94 RSC.
- R. Nishiyabu, H. Kobayashi and Y. Kubo, RSC Adv., 2012, 2, 6555–6561 RSC.
- Y. Guan and Y. J. Zhang, Chem. Soc. Rev., 2013, 42, 8106–8121 RSC.
- R. W. Guo, Q. Su, J. W. Zhang, A. J. Dong, C. G. Lin and J. H. Zhang, Biomacromolecules, 2017, 18, 1356–1364 CrossRef CAS PubMed.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef CAS PubMed.
- H. Zhu, J. L. Fan, S. L. Zhang, J. F. Cao, K. D. Song, D. Ge, H. J. Dong, J. Y. Wang and X. J. Peng, Biomater. Sci., 2014, 2, 89–97 RSC.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- E. J. Kim, S. Bhuniya, H. Lee, H. M. Kim, C. Cheong, S. Maiti, K. S. Hong and J. S. Kim, J. Am. Chem. Soc., 2014, 136, 13888–13894 CrossRef CAS PubMed.
- M. L. Odyniec, H. H. Han, J. E. Gardiner, A. C. Sedgwick, X. P. He, S. D. Bull and T. D. James, Front. Chem., 2019, 7 DOI:10.3389/fchem.2019.00775.
- Y. Qiu and K. Park, Adv. Drug Delivery Rev., 2001, 53, 321–339 CrossRef CAS PubMed.
- Y. Lee, K. H. Choi, K. M. Park, J. M. Lee, B. J. Park and K. D. Park, ACS Appl. Mater. Interfaces, 2017, 9, 16891–16900 Search PubMed.
- C. H. Ren, L. P. Chu, F. Huang, L. J. Yang, H. R. Fan, J. F. Liu and C. H. Yang, RSC Adv., 2017, 7, 1313–1317 RSC.
- F. Liu, L. B. Bai, H. L. Zhang, H. Z. Song, L. D. Hu, Y. G. Wu and X. W. Ba, ACS Appl. Mater. Interfaces, 2017, 9, 31626–31633 CrossRef CAS PubMed.
- S. Granick and L. Michaelis, J. Am. Chem. Soc., 1947, 69, 2983–2986 CrossRef CAS PubMed.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radical Biol. Med., 2009, 47, 1401–1407 CrossRef CAS PubMed.
- A. C. Sedgwick, W. T. Dou, J. B. Jiao, L. L. Wu, G. T. Williams, A. T. A. Jenkins, S. D. Bull, J. L. Sessler, X. P. He and T. D. James, J. Am. Chem. Soc., 2018, 140, 14267–14271 CrossRef CAS PubMed.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2017, 53, 12822–12825 RSC.
- H. H. Han, A. C. Sedgwick, Y. Shang, N. Li, T. T. Liu, B. H. Li, K. Q. Yu, Y. Zang, J. T. Brewster, M. L. Odyniec, M. Weber, S. D. Bull, J. Li, J. L. Sessler, T. D. James, X. P. He and H. Tian, Chem. Sci., 2020, 11, 1107–1113 RSC.
- B. C. Dickinson, C. Huynh and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 5906–5915 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc01904f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |