 Open Access Article
Open Access ArticleFacile assembly of three cycloalkyne-modules onto a platform compound bearing thiophene S,S-dioxide moiety and two azido groups†
Tomohiro
Meguro
,
Yuki
Sakata
,
Takamoto
Morita
,
Takamitsu
Hosoya
 and
Suguru
Yoshida
and
Suguru
Yoshida
 *
*
Laboratory of Chemical Bioscience, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University (TMDU), 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan. E-mail: s-yoshida.cb@tmd.ac.jp
First published on 19th March 2020
Abstract
An efficient method to assemble three cycloalkyne-modules onto a platform compound bearing a thiophene S,S-dioxide moiety and two azido groups has been developed. The sequential reactions without catalysis or additives enabled the facile preparation of trifunctional molecules by a simple procedure. One-pot assembly was also achieved using the platform and three cycloalkynes.
Modular synthesis by consecutive reactions onto a platform molecule has gained attention as a facile method for preparing a wide variety of products from diverse modules.1,2 On the basis of the remarkable achievements by recently emerging click chemistry3 including copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC)4 and strain-promoted azide–alkyne cycloaddition (SPAAC),5,6 efficient assembly of modules by multi-click chemistry using well-designed platform molecules has been accomplished (Fig. 1). For example, Jiráček and coworkers achieved a triple-CuAAC reaction using platform 1 bearing terminal, triethylsilyl, and triisopropylsilyl alkynes through selective desilylprotonation (Fig. 1A).7 Triple-conjugation by tetrazine–norbornene ligation, CuAAC, and thiol–maleimide reaction using platform 3 developed by Knall and coworkers allowed for assembling three-types of modules (Fig. 1B).8 Recently, we also developed a tris(triazole) formation method by three sequential reactions using platform 5 having three-types of azido groups. This method enabled to assemble three types of azidophiles having functional groups, such as HaloTag ligand, fluorescent, and biotin moieties (Fig. 1C).9 Despite these continuous efforts, an ideal method for assembling modules onto a platform molecule under catalysis-free conditions is not easy to develop due to the limited methods for reliable conjugation10 and difficulties to synthesize a platform molecule bearing discriminatable reactive groups. Herein, we describe an efficient method for highly selective triple-conjugation of three cycloalkynes onto a newly designed platform bearing discriminatable thiophene S,S-dioxide moiety and two azido groups (Fig. 1D).
 | ||
| Fig. 1 Methods to assemble three modules onto a platform molecule. (A) Jiráček's work. (B) Knall's work. (C) Our previous work. (D) This work. | ||
Focusing on the remarkable reactivities of cycloalkynes allowing for efficient conjugation under mild conditions without catalysis, we at first explored ynophiles with discriminatable reactivity (Fig. 2). Based on our previous report that 2,3,4,5-tetrachlorothiophene S,S-dioxide slowly reacted with dibenzo-fused cyclooctyne 8 at room temperature,11,12 selective SPAAC reaction of cyclooctyne 8 with platform 7 proceeded smoothly at the benzyl azido group to provide triazole 9 leaving thiophene dioxide moiety untouched (Fig. 2A). We then envisioned that a bulky alkyl azide can remain intact in the SPAAC reaction of the benzyl azido group,13,14 although the difference of the clickability between benzyl azide and phenyl azide in the SPAAC reaction was not satisfied.2c,6f Thus, a competitive SPAAC reaction of cyclooctyne 8 with an equimolar mixture between benzyl azide (10a) and bulky alkyl azide 10b, 10c, or 10d was examined (Fig. 2B). It was shown that the SPAAC of benzyl azide (10a) took place selectively when using 1-adamantyl azide (10d), while the selectivity was not satisfiable in the reaction of a mixture between secondary azide 10b or 10c and benzyl azide (10a). Furthermore, selective [2+3] cycloaddition between 1-adamantyl azide (10d) and thiophene dioxide 12 was accomplished by treatment with dibenzo-fused cyclooctyne 86d or 4,8-diazacyclononyne (DACN) derivative 146g at room temperature without providing undesired products by the reaction of thiophene dioxide (Fig. 3). These results clearly showed that selective reactions of cycloalkynes with a platform having benzyl azide, 1-adamantyl azide, and thiophene dioxide moieties would proceed efficiently to furnish conjugated products without any undesired side products.
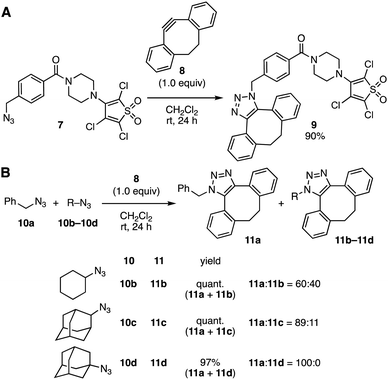 | ||
| Fig. 2 SPAAC reactions between azides and dibenzo-fused cyclooctyne 8. (A) Selective reaction of platform 7. (B) Competition experiments using azides and cyclooctyne 8. | ||
 | ||
| Fig. 3 Competition experiments treating a mixture of azide 10d and thiophene dioxide 12 with cyclooctyne 8 or 14. | ||
Having three discriminatable ynophiles in hand, we designed platform molecule 24 possessing these ynophilic moieties (Fig. 4). The synthesis of platform 24 was achieved from simple building blocks 17, 19, 21, and 23. Indeed, selective reduction at the aromatic azido group15 of diazide 1716 followed by amide formation using 3-azidoadamantane carboxylic acid (19) afforded diazide 20 in good yield. Then, transformations of methoxycarbonyl group to (4-(tert-butoxycarbonyl)piperazino)carbonyl group and subsequent removal of the tert-butoxycarbonyl group and substitution reaction with 2,3,4,5-tetrachlorothiophene S,S-dioxide (23) successfully provided platform 24 leaving two azido groups untouched.
 | ||
| Fig. 4 Synthesis of platform 24. EDC = 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide. DMAP = 4-dimethylaminopyridine. | ||
Consecutive reactions using platform 24 with three cycloalkynes 8, 14, and 25 enabled efficient assembly to afford the desired conjugate 26 in a highly selective manner (Fig. 5). Indeed, SPAAC reaction of platform 24 with dibenzo-fused cyclooctyne 8 proceeded selectively as expected without reactions of 1-adamantyl azide or thiophene dioxide moieties (Fig. 5A). The remaining tertiary azide smoothly reacted with DACN derivative 14 with gentle heating and subsequent reaction between thiophene dioxide and bicyclo[6.1.0]non-4-yne (BCN) derivative 256e took place efficiently.17 Thus, facile assembly by three consecutive catalysis-free reactions was achieved by the simple procedure without yielding any side products. Furthermore, one-pot assembly of the three components onto platform 24 successfully proceeded by sequential addition of cycloalkynes 8, 14, and 25 (Fig. 5B).18
To showcase the simplicity and efficiency of the method for preparing conjugates from cycloalkyne-modules, synthesis of trifunctional molecule 29 was demonstrated by assembling three functional cycloalkynes 28a–c onto platform 24 (Fig. 6). Three types of functional cycloalkynes 28a–c having a fluorescent rhodamine, biotinyl group, and ligand moiety for HaloTag, were successfully synthesized by CuAAC reactions on the basis of azide-to-cycloalkyne switching approach using the corresponding diynes 27 through transient protection of cycloalkyne moieties with copper (Fig. 6A and B).19 Then, these cycloalkyne-modules, azadibenzocyclooctyne (DIBAC) derivative 28a, DACN derivative 28b, and BCN derivative 28c, were efficiently assembled to platform 24 in excellent yields as a mixture of regioisomers without damaging three functional moieties (Fig. 6C). Monitoring each step by HPLC analysis clearly showed the significant efficiency of this assembling method.17
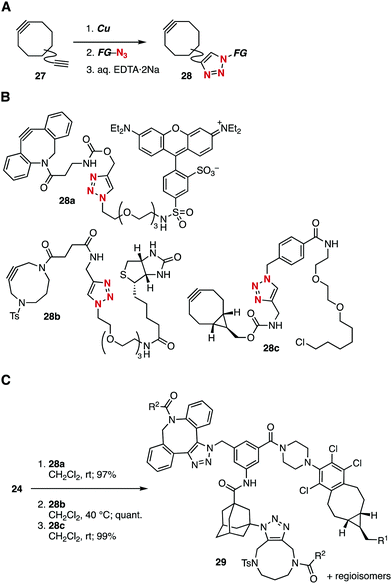 | ||
| Fig. 6 Assembly of three cycloalkyne-modules. (A) Synthesis of cycloalkyne-modules 28. (B) Structures of cycloalkyne-modules 28. (C) Assembly of functional cycloalkynes 28 onto platform 24. See the ESI† for the detailed structure of product 29. EDTA·2Na = ethylenediamine-N,N,N′,N′-tetraacetic acid disodium salt. | ||
In summary, we have developed an efficient method to assemble three cycloalkyne-modules using a platform compound having thiophene S,S-dioxide moiety and two azido groups. Since a broad range of functional cycloalkyne-modules are easily prepared by azide-to-cycloalkyne switching approach from easily available functional azides, this simple assembly method would serve in the preparation of various trifunctional molecules from easily available azide-modules. Further studies involving development of a thiophene dioxide-selective reaction of platform molecule 24 and application of this method are underway in our laboratory.
This work was supported by JSPS KAKENHI Grant Numbers JP19K05451 (C; S. Y.), JP18J11113 (JSPS Research Fellow; T. M.), JP18H02104 (B; T. H.), and JP18H04386 (Middle Molecular Strategy; T. H.); the Naito Foundation (S. Y.); the Japan Agency for Medical Research and Development (AMED) under Grant Number JP19am0101098 (Platform Project for Supporting Drug Discovery and Life Science Research, BINDS); and the Cooperative Research Project of Research Center for Biomedical Engineering.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A.-C. Knall and C. Slugovc, Chem. Soc. Rev., 2013, 42, 5131 RSC; (b) Z.-J. Zheng, D. Wang, Z. Xu and L.-W. Xu, Beilstein J. Org. Chem., 2015, 11, 2557 CrossRef CAS PubMed; (c) S. Yoshida, Bull. Chem. Soc. Jpn., 2018, 91, 1293 CrossRef CAS; (d) S. Yoshida, Org. Biomol. Chem., 2020, 18, 1550 RSC.
- (a) I. E. Valverde, A. F. Delmas and V. Aucagne, Tetrahedron, 2009, 65, 7597 CrossRef CAS; (b) B. C. Sanders, F. Friscourt, P. A. Ledin, N. E. Mbua, S. Arumugam, J. Guo, T. J. Boltje, V. V. Popik and G.-J. Boons, J. Am. Chem. Soc., 2011, 133, 949 CrossRef CAS PubMed; (c) J. Dommerholt, O. van Rooijen, A. Borrmann, C. F. Guerra, F. M. Bickelhaupt and F. L. van Delft, Nat. Commun., 2014, 5, 5378 CrossRef CAS PubMed; (d) P. R. Werkhoven, H. van de Langemheen, S. van der Wal, J. A. W. Kruijtzer and R. M. J. Liskamp, J. Pept. Sci., 2014, 20, 235 CrossRef CAS PubMed; (e) M. Robert, J. Vallée, P. Majkut, D. Krause, M. Gerrits and C. P. R. Hackenberger, Chem. – Eur. J., 2015, 21, 970 CrossRef PubMed; (f) R. R. Ramsubhag and G. B. Dudley, Org. Biomol. Chem., 2016, 14, 5028 RSC; (g) D. A. Sutton, S.-H. Yu, R. Steet and V. V. Popik, Chem. Commun., 2016, 52, 553 RSC; (h) T. Meguro, N. Terashima, H. Ito, Y. Koike, I. Kii, S. Yoshida and T. Hosoya, Chem. Commun., 2018, 54, 7904 RSC; (i) T. Meguro, S. Yoshida, K. Igawa, K. Tomooka and T. Hosoya, Org. Lett., 2018, 20, 4126 CrossRef CAS PubMed; (j) T. Yokoi, H. Tanimoto, T. Ueda, T. Morimoto and K. Kakiuchi, J. Org. Chem., 2018, 83, 12103 CrossRef CAS PubMed; (k) T. Yokoi, T. Ueda, H. Tanimoto, T. Morimoto and K. Kakiuchi, Chem. Commun., 2019, 55, 1891 RSC.
- (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS; (b) C. S. McKay and M. G. Finn, Chem. Biol., 2014, 21, 1075 CrossRef CAS PubMed; (c) J. Lahann, Click Chemistry for Biotechnology and Materials Science, John Wiley & Sons, West Sussex, 2009 CrossRef.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed.
- (a) M. F. Debets, C. W. J. van der Doelen, F. P. J. T. Rutjes and F. L. van Delft, ChemBioChem, 2010, 11, 1168 CrossRef CAS PubMed; (b) J. C. Jewett and C. R. Bertozzi, Chem. Soc. Rev., 2010, 39, 1272 RSC; (c) E. M. Sletten and C. R. Bertozzi, Acc. Chem. Res., 2011, 44, 666 CrossRef CAS PubMed; (d) S. Arumugam, S. V. Orski, N. E. Mbua, C. McNitt, G.-J. Boons, J. Locklin and V. V. Popik, Pure Appl. Chem., 2013, 85, 1499 CAS; (e) J. Dommerholt, F. P. J. T. Rutjes and F. L. van Delft, Top. Curr. Chem., 2016, 374, 16 CrossRef PubMed.
- (a) N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046 CrossRef CAS PubMed; (b) J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793 CrossRef CAS PubMed; (c) J. C. Jewett, E. M. Sletten and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 3688 CrossRef CAS PubMed; (d) X. Ning, J. Guo, M. A. Wolfert and G.-J. Boons, Angew. Chem., Int. Ed., 2008, 47, 2253 CrossRef CAS PubMed; (e) J. Dommerholt, S. Schmidt, R. Temming, L. J. A. Hendriks, F. P. J. T. Rutjes, J. C. M. van Hest, D. J. Lefeber, P. Friedl and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 9422 CrossRef CAS PubMed; (f) S. Yoshida, A. Shiraishi, K. Kanno, T. Matsushita, K. Johmoto, H. Uekusa and T. Hosoya, Sci. Rep., 2011, 1, 82 CrossRef PubMed; (g) R. Ni, N. Mitsuda, T. Kashiwagi, K. Igawa and K. Tomooka, Angew. Chem., Int. Ed., 2015, 54, 1190 CrossRef CAS PubMed; (h) S. Yoshida, J. Tanaka, Y. Nishiyama, Y. Hazama, T. Matsushita and T. Hosoya, Chem. Commun., 2018, 54, 13499 RSC.
- (a) V. Vaněk, J. Pícha, B. Fabre, M. Buděšínský, M. Lepšík and J. Jiráček, Eur. J. Org. Chem., 2015, 3689 CrossRef; (b) B. Fabre, J. Pícha, V. Vaněk, I. Selicharová, M. Chrudinová, M. Collinsová, L. Žáková, M. Buděšínský and J. Jiráček, ACS Comb. Sci., 2016, 18, 710 CrossRef CAS PubMed; (c) B. Fabre, J. Pícha, V. Vaněk, M. Buděšínský and J. Jiráček, Molecules, 2015, 20, 19310 CrossRef CAS PubMed.
- A.-C. Knall, M. Hollauf, R. Saf and C. Slugovc, Org. Biomol. Chem., 2016, 14, 10576 RSC.
- S. Yoshida, K. Kanno, I. Kii, Y. Misawa, M. Hagiwara and T. Hosoya, Chem. Commun., 2018, 54, 3705 RSC.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974 CrossRef CAS PubMed.
- T. Meguro, S. Yoshida and T. Hosoya, Chem. Lett., 2017, 46, 1137 CrossRef CAS.
- Recent reports on transformations using thiophene S,S-dioxides, see: (a) W. Wang, X. Ji, Z. Du and B. Wang, Chem. Commun., 2017, 53, 1370 RSC; (b) S. Suzuki, T. Asako, K. Itami and J. Yamaguchi, Org. Biomol. Chem., 2018, 16, 3771 RSC; (c) T. Asako, S. Suzuki, K. Itami, K. Muto and J. Yamaguchi, Chem. Lett., 2018, 47, 968 CrossRef CAS.
- Selective click chemistry using bulky azides was presented in 2013, see: T. Morita, S. Yoshida, A. Shiraishi and T. Hosoya, 24th Symposium on Physical Organic Chemistry, Tokyo, Japan, September 5, 2013, Abstr., No. 1P038.
- For recent examples of selective click conjugation using bulky azides, see: (a) N. Münster, P. Nikodemiak and U. Koert, Org. Lett., 2016, 18, 4296 CrossRef PubMed; (b) D. Svatunek, N. Houszka, T. A. Hamlin, F. M. Bickelhaupt and H. Mikula, Chem. – Eur. J., 2019, 25, 754 CrossRef CAS.
- T. Meguro, S. Yoshida and T. Hosoya, Chem. Lett., 2017, 46, 473 CrossRef CAS.
- S. Yoshida, Y. Misawa and T. Hosoya, Eur. J. Org. Chem., 2014, 3991 CrossRef CAS.
- While thiophene S,S-dioxides smoothly reacted with BCN-type cycloalkynes such as 25, the reactivity of thiophene S,S-dioxide with cycloalkynes 8 and 14 was low as reported in ref. 11.
- See the ESI† for details.
- (a) S. Yoshida, Y. Hatakeyama, K. Johmoto, H. Uekusa and T. Hosoya, J. Am. Chem. Soc., 2014, 136, 13590 CrossRef CAS PubMed; (b) S. Yoshida, T. Kuribara, H. Ito, T. Meguro, Y. Nishiyama, F. Karaki, Y. Hatakeyama, Y. Koike, I. Kii and T. Hosoya, Chem. Commun., 2019, 55, 3556 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization for new compounds including NMR spectra. See DOI: 10.1039/d0cc01810d |
| This journal is © The Royal Society of Chemistry 2020 |

