Open Access Article
Open Access ArticleSynthesis of γ-cyclodextrin substituted bis(acyl)phosphane oxide derivative (BAPO-γ-CyD) serving as multiple photoinitiator and crosslinking agent†
Andrea
Cosola
 a,
Riccardo
Conti
a,
Riccardo
Conti
 b,
Vijay Kumar
Rana
bc,
Marco
Sangermano
b,
Vijay Kumar
Rana
bc,
Marco
Sangermano
 a,
Annalisa
Chiappone
a,
Annalisa
Chiappone
 a,
Joëlle
Levalois-Grützmacher
bd and
Hansjörg
Grützmacher
a,
Joëlle
Levalois-Grützmacher
bd and
Hansjörg
Grützmacher
 *b
*b
aDISAT, Politecnico di Torino, Corso Duca degli Abruzzi 21, 10129 Torino, Italy
bDepartment of Chemistry and Applied Biosciences, ETH Zurich, 8093 Zurich, Switzerland. E-mail: hgruetzmacher@ethz.ch
cDepartment of Chemistry, Melville Laboratory for Polymer Synthesis, University of Cambridge, Cambridge CB2 1EW, UK
dUniversité des Antilles, FWI, 97110 Guadeloupe, France
First published on 25th March 2020
Abstract
A new multi-photoactive γ-cyclodextrin substituted bis(acyl)phosphane oxide derivative (BAPO-γ-CyD) was successfully prepared via a convergent synthesis using a phospha-Michael-addition, as confirmed by 1H-, 13C-, 31P-NMR and IR spectroscopy and mass spectrometry. Kinetic studies carried out by photo-DSC and photo-rheology demonstrated its outstanding efficiency as a photoinitiator for free-radical polymerization. Remarkably, it is found that BAPO-γ-CyD also acts as a crosslinking agent to convert monofunctional methacrylate monomers into self-standing thermosetting networks with extensive swelling capability in water.
Cyclodextrins (CyDs), first described by A. Villiers in 1891,1 comprise a family of cyclic oligosaccharides obtained via enzymatic degradation of starch by the action of glucosyl transferase (CGTase).2 These molecules consist of either six, seven or eight D-glucopyranose subunits joined together by α-(1,4)-linkages and arranged in torus-shaped macro-cycles, named α-CyD, β-CyD and γ-CyD, respectively. These compounds show a hydrophilic outer surface and a lipophilic inner cavity.3–6 Due to their structural conformation and amphiphilic behaviour, CyDs are typically used to form inclusion complexes with hydrophobic guests having a suitable molecular size.7–9 Cyclodextrins are very versatile building blocks because their hydroxyl groups, located on the narrow as well as the wide rim of the cavity [primary (OH6) and secondary sites (OH2/OH3) respectively], are rather easily chemically modified. Consequently, a wide range of functionalized CyDs for a broad scope of applications have been synthesized in recent years.10,11
Photoinitiators (PIs) play a key role in photopolymerisations, generating free radicals upon light irradiation that rapidly react with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of, for example, acrylic monomers. Among all photoinitiating systems known so far, bis(acyl)phosphane oxides (R1PO(COR2)2 = BAPOs) are very popular because they are highly reactive Norrish type I PIs used in several industrial processes for coatings, inks, adhesives, and dental materials.12–15 More recently, several P-functionalized BAPO derivatives were synthesized and successfully tested for various applications including the synthesis of star-shaped polymers, 3D-printing of hydrogels, and Janus-type particles.16–22
C double bonds of, for example, acrylic monomers. Among all photoinitiating systems known so far, bis(acyl)phosphane oxides (R1PO(COR2)2 = BAPOs) are very popular because they are highly reactive Norrish type I PIs used in several industrial processes for coatings, inks, adhesives, and dental materials.12–15 More recently, several P-functionalized BAPO derivatives were synthesized and successfully tested for various applications including the synthesis of star-shaped polymers, 3D-printing of hydrogels, and Janus-type particles.16–22
Here we report the preparation of a γ-cyclodextrin derivative which was decorated via the hydroxyl groups with bis(acyl)phosphaneoxide (BAPO) groups at the upper and lower rim to give a multi-active photoinitiator. This new molecule acts as highly efficient photoinitiator for free-radical polymerisation from which up to 20 radical sites can be generated and which therefore also acts as crosslinking agent to convert monofunctional methacrylate monomers into a 3D-network without any further additives.
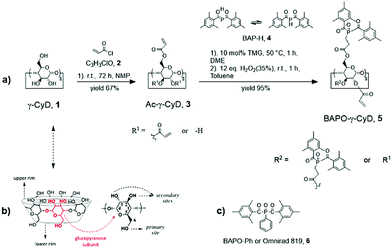
The target molecule BAPO-γ-CyD (5) was synthesized following a three-step reaction as shown in Fig. 1a.
We chose γ-cyclodextrin (1) as platform molecule because we speculated that it offers the possibility to bind a maximum of photoactive groups –PO(COMes)2 which are rather bulky (about 410 Å3). Acrylated-γ-cyclodextrin (3) was prepared following a slightly modified synthetic route reported by Gil et al.23 which consists in adding acryloyl chloride (2) dropwise to a N-methyl pyrrolidone solution of γ-cyclodextrin (1) under inert conditions. By slowly dropping the reaction mixture into deionized water (DI-H2O), 3 is precipitated as a white powder in 67% yield. The identity of the product was confirmed by NMR and IR (Fig. S1–S3, ESI†) which agree with the data reported in the literature. γ-CyD (1) has 24 OH groups in total (8 primary at the lower rim and 16 secondary ones at the upper rim). The MALDI-MS spectrum of dry 3 (Fig. S4, ESI†) shows that on average 21 hydroxyl groups were acrylated indicating that substitution occurs at both sites.
In a straightforward reaction, bis(2,4,6-trimethylbenzoyl)hydrogenphosphane (BAP-H, 4), which is in equilibrium with its tautomer, (MesCO)P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OH)Mes,18,19,24 was added in the next step stereoselectively to the terminal carbon center of the C
C(OH)Mes,18,19,24 was added in the next step stereoselectively to the terminal carbon center of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of 3 (phospha-Michael addition).25–31 Subsequent oxygenation with aqueous H2O2 leads to the phosphaneoxide BAPO-γ-CyD (5) in high isolated yield (>95%). Two closely lying 13P{1H} NMR signals at δ = 25.04 and 25.51 ppm (Fig. S5, ESI†) indicate that both vinyl acceptor groups at the bottom and upper rim of 3 react with 4 and are decorated with BAPO units which is further proven by 1H-NMR, 13C{1H}-NMR and ATR-FTIR spectra. In particular, even if the spectra of 5 are quite complex, the characteristic 1H chemical shifts for the methyl protons of the mesityl groups (CH3Mes) and the aromatic protons (HarMes) are clearly identified at δ = 2.13–2.23 ppm and δ = 6.77 ppm respectively (Fig. S6, ESI†), as well as the 13C shifts for the carbonyl carbons (COMes) at 216.5 ppm (Fig. S7, ESI†), in good agreement with those of other BAPO derivatives.16–22 Likewise, the ATR-FTIR spectrum of 5 shows the typical stretching vibrations of ν(COMes) and ν(P
C double bonds of 3 (phospha-Michael addition).25–31 Subsequent oxygenation with aqueous H2O2 leads to the phosphaneoxide BAPO-γ-CyD (5) in high isolated yield (>95%). Two closely lying 13P{1H} NMR signals at δ = 25.04 and 25.51 ppm (Fig. S5, ESI†) indicate that both vinyl acceptor groups at the bottom and upper rim of 3 react with 4 and are decorated with BAPO units which is further proven by 1H-NMR, 13C{1H}-NMR and ATR-FTIR spectra. In particular, even if the spectra of 5 are quite complex, the characteristic 1H chemical shifts for the methyl protons of the mesityl groups (CH3Mes) and the aromatic protons (HarMes) are clearly identified at δ = 2.13–2.23 ppm and δ = 6.77 ppm respectively (Fig. S6, ESI†), as well as the 13C shifts for the carbonyl carbons (COMes) at 216.5 ppm (Fig. S7, ESI†), in good agreement with those of other BAPO derivatives.16–22 Likewise, the ATR-FTIR spectrum of 5 shows the typical stretching vibrations of ν(COMes) and ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1678 cm−1 and 1143 cm−1 respectively (Fig. S8, ESI†).
O) at 1678 cm−1 and 1143 cm−1 respectively (Fig. S8, ESI†).
Again MALDI-MS spectra were used to determine the degree of functionalisation obtained in 5. The results revealed that a full addition to all vinyl groups in 3 cannot be achieved likely due to the steric hindrance. Instead, a limit of about 10 BAPO units per molecule can be reached at best even under forcing reaction conditions using an excess of BAP-H 4. Thereafter, only 11 equivalents of BAP-H were used for the synthesis to limit the formation of undesired by-products which stem from the unreacted Michael donor 4. As confirmed by 1H-NMR, 13C{1H}-NMR and ATR-FTIR, about half of the vinyl groups in 3 were phosphorylated while the other half remains intact. This should make 5 a promising multi-photoactive crosslinking agent via both, the BAPO and acrylate functions. The UV/Vis spectrum of 5 [c = 0.1 × 10−3 mol L−1 in dichloromethane (DCM)] was measured and compared to that of commercial phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide [BAPO-Ph or Omnirad 819 (6)] (Fig. 2). BAPO-γ-CyD (5) shows a long-wave absorption band above 360 nm corresponding to the n → π* excitation of the CO chromophore, similar to that of 6 and in line with the typical values reported for BAPOs.16 Furthermore, 5 shows a considerably stronger absorption than equimolar amount of 6, while, raising the concentration of 6 by a factor of ten (c = 1 × 10−3 mol L−1), the obtained spectra appear quite similar. The comparison of the molar extinction coefficients (ε) further confirms the effective grafting of about 10 BAPO units, since 5 exhibits an ε almost ten times higher (ε5 = 5484 L mol−1 cm−1) than 6 (ε6 = 530 L mol−1 cm−1).
To investigate the suitability of 5 as photoinitiator for free radical polymerisation, photo-differential scanning calorimetry (photo-DSC) and photo-rheology were carried out in standard formulations containing 1,6 hexanediol diacrylate (HDDA) as monomer. Omnirad 819 (6) was used again as reference PI. Photocuring was carried out under a nitrogen atmosphere to limit oxygen inhibition, using a Hamamatsu LC8 lamp with a cutoff filter under 400 nm and very low intensity (0.6 mW cm−2) as light source. From the resulting DSC curves, the heat flow, the time to reach the onset of polymerisation (tonset) and the maximum of heat flux (tmax), the double bond conversion (DBC), and the rate of conversion (Rp) can be determined. The photo-DSC plots (Fig. 3) revealed that, when equimolar PI concentrations (0.2 mM) are compared, the multi-active PI 5 (0.2 mM, 0.11 wt%) is much more reactive than 6 (0.2 mM, 0.008 wt%), as indicated by short tonset < 4 s, and tmax = 34 s and fast Rp = 0.027 s−1 leading to a final DBC of HDDA of 67%. Compared to this, the performance of 6 is much lower with long tonset > 20 s, and tmax = 120 s, slow Rp,max = 0.007 s−1 and a total conversion of the monomer below 50%.
We performed experiments in which the concentration of the photoinitiator was adjusted such that the formulations contained equimolar amounts of photoactive groups. Under the assumption that BAPO-γ-CyD (5) contains ten BAPO units on average, the concentration of BAPO-Ph (6) was raised to 2 mM. Under these conditions, using a ten times higher concentration of 6 (2 mM, 0.08 wt%), an almost equal activity (tonset < 5 s, tmax = 31 s, Rp,max = 0.029 s−1 and DBC = 65%) comparable to the one observed with 5 (0.2 mM, 0.11 wt%) is observed. The photo-rheology curves shown in Fig. 4 allow to evaluate the shear storage modulus (G′) variations versus the irradiation time. These are in agreement with photo-DSC data. Using HDDA formulations as described above and employing PI concentrations with equimolar amounts of radically cleavable groups, that is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 = 1
6 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, almost identical delay times (td, i.e. irradiation time required to induce chemical crosslinking) were observed (td_5(0.2mM) = 8.75 ± 0.5 s; td_6(2mM) = 7.50 ± 0.6 s).
10, almost identical delay times (td, i.e. irradiation time required to induce chemical crosslinking) were observed (td_5(0.2mM) = 8.75 ± 0.5 s; td_6(2mM) = 7.50 ± 0.6 s).
Also, the slopes of the curves are very similar (ΔG′/Δt > 24 kPa s−1), suggesting comparable curing rates. But for formulations that contain equimolar PI concentrations, we observe that prolonged irradiation times are required in order to start the curing process using 6 (td_6(0.2mM) > 90 s) and the photopolymerisation proceeds significantly slower (ΔG′/Δt > 0.06 kPa s−1).
These results show that BAPO-γ-CyD can be used as photoinitiator at much lower molar concentrations without any loss of activity. This feature is of high interest for practical applications in which a PI with a high-molecular weight must be applied in order to guarantee low migration in the formulation without loss of photoactivity. As stated above, with ten photoactive groups and the residual acrylate functions, BAPO-γ-CyD (5) should not only behave as photoinitiator but also as cross-linking agent.16 This property was tested using a mono acrylate as monomer. As reference, the monofunctional BAPO-Ph (6) was used again in ten times higher concentration for comparison. Four different formulations were prepared by dissolving either 5 or 6 in poly(ethylene glycol) methyl ether methacrylate (PEGMEM, Mn = 500) as monomer. The curing process was followed via photo-rheology (Fig. 5). The curves clearly show that shorter delay times (td < 40 s), and faster kinetics were obtained for polymerisation with 5 as PI [formulation II (0.2 mM, 0.11 wt%), III (2 mM, 1.08 wt%), and IV (20 mM, 9.8 wt%), Table 1] which also leads to materials P II, P III, P IV with higher storage moduli upon curing compared to the polymeric material P I obtained using 6 [formulation I (2 mM, 0.077 wt%), Table 1]. Increasing the concentration of 5, leads to even faster curing rates and the formulations reached higher G′ values. These results demonstrate that BAPO-γ-CyD has not only a positive effect on the kinetics but also on the final mechanical properties of the samples. This strongly suggests that covalent cross-linked networks are obtained, in which polymer chains composed from PEGMEM repeating units are cross-linked via phosphorylated γ-cyclodextrin units. The cross-linking can occur either via the PO-γ-CyD groups, which are generated under photolysis, or via the residual acrylate groups in 5. On the contrary, when the mono-functional 6 is used as PI under otherwise identical conditions using formulation I, the variation of G′ under irradiation indicates after long exposure times (td > 90 s) the generation of a viscous and sticky paste as a result of an increase of the molecular weight of the growing thermoplastic polymer. This is typical for a linear polymerisation process, rather than to the generation of a covalent cross-linked network. Gel content (GC) measurements also confirmed that PEGMEM can be converted into a thermoset polymer just using BAPO-γ-CyD, thus proving its superior performance over standard molecular PIs.
 | ||
| Fig. 5 Photo-rheology curves carried out at r.t. of formulations I, II, III and IV; and picture of a sample P II obtained from photopolymerisation of II, before and after swelling in water at r.t. | ||
| Formulation | BAPO [mM] | BAPO-γ-CyD [mM] | G′ (kPa) | SWeq. (%) | EWC (%) | GC (%) |
|---|---|---|---|---|---|---|
| I | 2.0 | — | 0.3 | — | — | — |
| II | — | 0.2 | 1.8 | 905 ± 7 | 90 ± 0.1 | 40 ± 2.5 |
| III | — | 2.0 | 2.9 | 767 ± 2 | 88 ± 0.1 | 78 ± 1.3 |
| IV | — | 20.0 | 6.7 | 662 ± 12 | 87 ± 0.6 | 77 ± 1.5 |
In a last set of experiments, small cylindrical samples were prepared by irradiating the formulations I–IV. The resulting polymeric materials were then immersed in water to separate the water-soluble (not cross-linked) and insoluble (cross-linked) fractions of the polymer. The resulting data are collected in Table 1. A sticky polymer P I is obtained upon irradiation of formulation I which rapidly dissolves in water indicating the absence of chemical crosslinking (GC = 0). On the contrary, the polymer samples P II, P III, and P IV prepared from formulations II, III and IV did not dissolve confirming the efficiency of crosslinking (GC up to 78%). In addition, P II–P IV extensively absorb water (Fig. 5) reaching high equilibrium swelling, SWeq. > 660%, and high water contents, EWC > 87%.
Remarkably, increasing the concentration of BAPO-γ-CyD 5 leads to a decrease of SWeq. (900% at 0.2 mM; 660% at 20 mM) while the storage modulus increases impressively from 1.8 kPa to 6.7 kPa. This result is in agreement with the assumption that 5 acts as both, photoinitiator and simultaneously as cross-linker, because a higher density of cross-linking points in the polymer network will lead to a mechanically more stable material less prone to swelling.
In conclusion, in this work a new multi-functional photoinitiator is presented which uses γ-cyclodextrin as platform. About 10 bis(acyl)phosphane oxide units could be successfully grafted to the lower and upper rim of the cyclodextrin as confirmed by 1H-, 13C-, 13P-NMR, IR and UV-vis spectroscopy as well as by mass spectrometry. In contrast to a poly-functional photoinitiator which consists of cellulose nanocrystals (CNC's) to which about 2000 BAPO units were grafted,16 BAPO-γ-CyD (5) is a molecularly well-defined initiator which is soluble in most common organic solvents. Photo-DSC and photo-rheology showed the outstanding high efficiency for free-radical polymerisation. Most importantly, BAPO-γ-CyD (5) stands out by its ability to serve simultaneously as photo-crosslinking agent which allows to convert a standard mono-functional monomer such as methacrylate into self-standing 3D-networks without any further additives. The resulting polymers show extensive swelling capabilities and the mechanical stability of the swollen polymers can be easily tuned by varying the concentration of 5 in the formulation.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Villiers, C. R. Acad. Sci., 1891, 112, 536–538 Search PubMed.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1753 CrossRef PubMed.
- E. M. M. Del Valle, Process Biochem., 2004, 39, 1033–1046 CrossRef.
- P. Jansook, N. Ogawa and T. Loftssonc, Int. J. Pharm., 2018, 535, 272–284 CrossRef CAS PubMed.
- N. Pedersen, J. B. Kristensen, G. Bauw, B. J. Ravoo, R. Darcy, K. L. Larsena and L. H. Pedersen, Asymmetry, 2005, 16, 615–622 CrossRef CAS.
- M. L. Bender and M. Komiyama, Cyclodextrin Chemistry, Springer Berlin Heidelberg, Berlin, Heidelberg, 1978, vol. 6 Search PubMed.
- S. V. Kurtov and T. Loftsson, Int. J. Pharm., 2013, 453, 167–180 CrossRef PubMed.
- M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875–1917 CrossRef CAS PubMed.
- H. Dodziuk, Cyclodextrins and their complexes: chemistry, analytical methods, applications, Wiley-VCH, 2006 Search PubMed.
- C. J. Easton and S. F. Lincoln, Modified cyclodextrins: scaffolds and templates for supramolecular chemistry, Imperial College Press, 1999 Search PubMed.
- J. Zhou and H. Ritter, Polym. Chem., 2010, 1, 1552–1559 RSC.
- K. Dietliker, A Compilation of Photoinitiators Commercially Available for UV Today, SITA Technology Ltd, London, UK, 2002 Search PubMed.
- J. V. Crivello and K. Dietliker, Photoinitiators for Free Radical Cationic & Anionic Photopolymerisation, Wiley, 2nd edn, 1999, vol. 168 Search PubMed.
- L. Gonsalvi and M. Peruzzini, Angew. Chem., Int. Ed., 2012, 51, 7895–7897 CrossRef CAS.
- H. Grützmacher, J. Geier, D. Stein, T. Ott, H. Schoenberg, R. H. Sommerlade, S. Boulmaaz, J. P. Wolf, P. Murer and T. Ulrich, Chimia, 2008, 62, 18–22 CrossRef.
- J. Wang, A. Chiappone, I. Roppolo, F. Shao, E. Fantino, M. Lorusso, D. Rentsch, K. Dietliker, C. F. Pirri and H. Grützmacher, Angew. Chem., Int. Ed., 2018, 57, 2353–2356 CrossRef CAS.
- A. Huber, A. Kuschel, T. Ott, G. Santiso-Quinones, D. Stein, J. Brauer, R. Kissner, F. Krumeich, H. Schonberg, J. Levalois-Grützmacher and H. Grützmacher, Angew. Chem., Int. Ed., 2012, 51, 4648–4652 CrossRef CAS.
- G. Müller, M. Zalibera, G. Gescheidt, A. Rosenthal, G. Santiso-Quinones, K. Dietliker and H. Grützmacher, Macromol. Rapid Commun., 2015, 36, 553–557 CrossRef.
- J. Wang, G. Siqueira, G. Müller, D. Rentsch, A. Huch, P. Tingaut, J. Levalois-Grützmacher and H. Grützmacher, Chem. Commun., 2016, 52, 2823–2826 RSC.
- M. Sangermano, M. Periolatto, M. Castellino, J. Wang, K. Dietliker, J. L. Grützmacher and H. Grützmacher, ACS Appl. Mater. Interfaces, 2016, 8, 19764–19771 CrossRef CAS.
- A. Eibel, M. Schmallegger, M. Zalibera, A. Huber, Y. Burkl, H. Grützmacher and G. Gescheidt, Eur. J. Inorg. Chem., 2017, 2469–2478 CrossRef CAS.
- A. Eibel, D. E. Fast, J. Sattelkow, M. Zalibera, J. Wang, A. Huber, G. Müller, D. Neshchadin, K. Dietliker, H. Plank, H. Grützmacher and G. Gescheidt, Angew. Chem., Int. Ed., 2017, 56, 14306–14309 CrossRef CAS.
- E. S. Gil, L. Xiu and T. L. Lowe, Biomacromolecules, 2012, 13, 3533–3541 CrossRef CAS.
- G. Becker, W. Becker, M. Schmidt, W. Schwarz and M. Westerhausen, Z. Anorg. Allg. Chem., 1991, 605, 7–23 CrossRef CAS.
- B. A. Trofimov, S. N. Arbuzova and N. K. Gusarova, Russ. Chem. Rev., 1999, 68, 215–227 CrossRef CAS.
- S. N. Arbuzova, N. K. Gusarova and B. A. Trofimov, ARKIVOC, 2006, 12–36 Search PubMed.
- D. S. Glueck, Chem. – Eur. J., 2008, 14, 7108–7117 CrossRef CAS.
- A. C. Chadwick, M. A. Heckenast, J. J. Rast, P. G. Pringke and H. A. Sparkes, Organometallics, 2019, 38, 3871 CrossRef CAS.
- M. M. Rauhut, I. Hechenbleikner, H. A. Currier, F. C. Schaefer and V. P. Wystrach, J. Am. Chem. Soc., 1959, 81, 1103 CrossRef CAS.
- I. Hechenbleikner and M. M. Rauhut, US Pat., 2822376, 1958 Search PubMed.
- R. B. King and P. N. Kapoor, J. Am. Chem. Soc., 1969, 91, 5191 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic protocols, experimental details, 1H, 13C and 31P NMR, ATR-FTIR, MALDI-MS and sample preparation. See DOI: 10.1039/d0cc01732a |
| This journal is © The Royal Society of Chemistry 2020 |