Mixed alkoxy/hydroxy 1,8-naphthalimides: expanded fluorescence colour palette and in vitro bioactivity†
Abstract
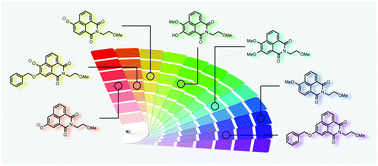
An efficient and functional group tolerant route to access hydroxy 1,8-naphthalimides has been used to synthesise a range of mono- and disubstituted hydroxy-1,8-naphthalimides with fluorescence emissions covering the visible spectrum. The dialkoxy substituted compounds prepared possess high quantum yields (up to 0.95) and long fluorescent lifetimes (up to 14 ns). The method has been used to generate scriptaid analogues that successfully inhibit HDAC6 in vitro with tubulin acetylation assays confirming that these compounds are more effective than tubastatin.
- This article is part of the themed collection: The Mechanics of Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...