A competitive self-powered sensing platform based on a visible light assisted zinc–air battery system†
Abstract
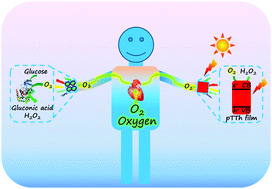
We developed a novel competitive self-powered sensing platform based on the discharge process of a visible light assisted zinc–air battery system for the detection of targets with a high open circuit potential signal (Eocpt = 1.5 V), which is a splendid signal among state-of-the-art self-powered sensing platforms.


 Please wait while we load your content...
Please wait while we load your content...