Pd-Catalyzed intermolecular C–H bond arylation reactions: effect of bulkiness of carboxylate ligands†
Abstract
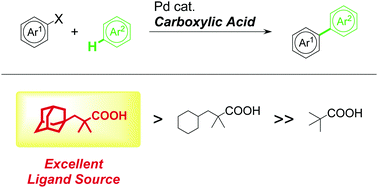
A bulky carboxylic acid bearing one 1-adamantylmethyl and two methyl substituents at the α-position is demonstrated to work as an efficient carboxylate ligand source in Pd-catalyzed intermolecular C(sp2)–H bond arylation reactions. The reactions proceeded smoothly under mild conditions, taking advantage of the steric bulk of the carboxylate ligands.


 Please wait while we load your content...
Please wait while we load your content...