Gold-catalyzed intermolecular [4+1] spiroannulation via site-selective aromatic C(sp2)–H functionalization and dearomatization of phenol derivatives†
Yongfeng
Li
a,
Zhiqiong
Tang
a,
Junliang
Zhang
 *b and
Lu
Liu
*b and
Lu
Liu
 *ac
*ac
aSchool of Chemistry and Molecular Engineering, East China Normal University, 500 Dongchuan Road, Shanghai, 200241, P. R. China. E-mail: lliu@chem.ecnu.edu.cn
bDepartment of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438, P. R. China. E-mail: junliangzhang@fudan.edu.cn
cShanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, East China Normal University, Shanghai, 200062, P. R. China
First published on 19th May 2020
Abstract
Herein, we have developed a novel and simple protocol to realize the C–H bond functionalization/dearomatization of naphthols and phenols with ortho-alkynylaryl-α-diazoesters under gold(I) catalysis. In this protocol, various spirocyclic molecules could be obtained in good yields with excellent chemo- and regio-selectivity and moderate to good diastereoselectivity.
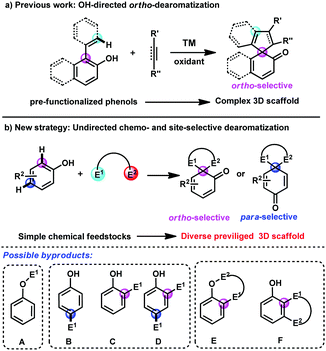
Phenols and naphthols are among the most readily available chemical feedstocks in industry and possess multiple reactive sites, which make them versatile building blocks for the synthesis of biologically active molecules, natural products and pharmaceuticals.1 Recently, the dearomatization reactions of phenols and naphthols have received great attention and emerged as one of the most efficient and straightforward approaches for the rapid construction of highly complex three-dimensional scaffolds from these planar aromatic molecules, which tremendously increases diversification in synthetic science.2,3 Several elegant examples in transition-metal-catalyzed dearomatization of phenols and naphthols have been developed,4,5 which provide an efficient route to construct the spriocycles.6 Nevertheless, most of these reactions still rely on intramolecular dearomatizations or intermolecular transformations of prefunctionalized starting materials. With the advance of transition-metal-catalysed C–H bond functionalization,7 a cooperative approach combining C–H bond functionalization and sequential dearomatization of arenes represents a step- and atom-economical tool to access the spirocycles. In this regard, Luan, You, Mascareñas and Gulías, Lam and others have reported several elegant transition-metal-catalyzed [3+2] and [2+2+1] spiroannulations of 2-naphthol or phenol derivatives and C–C multiple bonds by aromatic or vinyl C(sp2)–H bond functionalization/dearomatization cascade reactions (Scheme 1a).8 However, there are still several limitations of these reactions, including the single reaction site (only at ortho-position of hydroxyl), narrow substrate scope and harsh conditions. Thus, the development of more novel approaches for diverse and straightforward C–H bond functionalization/dearomatization reactions of commercially available phenols and naphthols is urgently desirable.
To address this daunting challenge, we hypothesized that the reaction of phenols and naphthols with bis-electrophiles may be achieved by the tandem C–H bond functionalization/dearomatization reactions, which will provide a straightforward approach to construct diverse spirocycles (Scheme 1b). However, this approach poses several considerable challenges. (1) The free phenolic hydroxyl group is active, which typically results in O–H bond functionalization with electrophile E1 rather than C–H bond functionalization (A, Scheme 1b). (2) Both the para- and ortho-sites on the phenyl ring are nucleophilic, typically leading to mixtures of ortho- and para-substituted products with various electrophiles (B–D, Scheme 1b). (3) After achieving the first site-selective C–H bond functionalization, how to control the following competing O–H substitution and C–H substitution is still challenging (E and F, Scheme 1b).
Recently, we have developed a gold-catalyzed9 site-selective aromatic C(sp2)–H bond functionalization of phenols, naphthols and toluene derivatives with diazo compounds.10 In addition, You and Zhang reported a highly efficient gold-catalyzed intramolecular dearomatization reaction of naphthols via 5-endo-dig cyclization.11 Thus, we envisioned that alkynyl diazo compound 1a should be a suitable bis-electrophile to achieve direct dearomatization of unmodified phenol derivatives by gold-catalyzed sequential C(sp2)–H functionalization/5-endo-dig carbocyclisation. It must be noted that the tandem C(sp2)–H functionalization/5-endo-dig cyclisation10j occurred when the internal alkynyl diazo compounds being similar to 1a reacted with phenols under the catalysis of the gold complex.
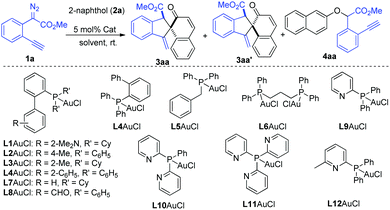
To test this hypothesis, our investigation began with the reaction of methyl o-alkynylaryl-α-diazoester 1a and β-naphthol 2a as the model substrates. The gold-complexes derived from PPh3 and (2,4-tBu2C6H3O)3P, which are commonly used in C(sp2)–H bond functionalization with diazo compounds, could not produce the desired product (Table 1, entries 1 and 2). IPrAuCl/AgNTf2 only gave a trace amount of the desired product. To our delight, the desired dearomatizative product 3aa/3aa′ was obtained in 75% total yield (51%/24%) with 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereo-selectivity and 12.5
1 diastereo-selectivity and 12.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chemo-selectivity in the presence of JohnPhosAuCl (5 mol%) and AgNTf2 (5 mol%) in DCM, in which the O–H bond insertion product 4aa was detected in 6% yield (Table 1, entry 4). Encouraged by this result, the screening of various phosphine ligands was conducted (Table 1, entries 5–17). Gratifyingly, 2-(diphenylphosphino)pyridine (L9) proved to be the most suitable ligand, affording the desired products 3aa/3aa′ in 94% total NMR yield with 4.5
1 chemo-selectivity in the presence of JohnPhosAuCl (5 mol%) and AgNTf2 (5 mol%) in DCM, in which the O–H bond insertion product 4aa was detected in 6% yield (Table 1, entry 4). Encouraged by this result, the screening of various phosphine ligands was conducted (Table 1, entries 5–17). Gratifyingly, 2-(diphenylphosphino)pyridine (L9) proved to be the most suitable ligand, affording the desired products 3aa/3aa′ in 94% total NMR yield with 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity and 47
1 diastereoselectivity and 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chemoselectivity (Table 1, entry 17). The counteranion effect of the halide scavenger was then investigated. The combination of L9AuCl and NaBArF displayed the highest reactivity (96% total yield), chemo-selectivity (2% of 4aa) and diastereo-selectivity (7.7
1 chemoselectivity (Table 1, entry 17). The counteranion effect of the halide scavenger was then investigated. The combination of L9AuCl and NaBArF displayed the highest reactivity (96% total yield), chemo-selectivity (2% of 4aa) and diastereo-selectivity (7.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) compared to those of AgNTf2 and AgBF4 (Table 1, entries 17–19). Further attempts to modify the pyridine-based phosphine ligands L10–L12 failed to improve the yield and diastereoselectivity (Table 1, entries 17–19). The solvent screening of, e.g., toluene and DCE could not give better results (Table 1, entries 23, 24 and Table S1 in the ESI†). The introduction of diazo compound 1a in one portion led to a lower yield (Table 1, entry 25). A series of other catalysts, such as Cu(OTf)2, AgOTf, and (C6F5)3B, which are commonly used in the transformation of diazo compounds, was also tested, and they showed very low catalytic activity and selectivity in this case (Table S1 in the ESI†). The structure and the relative configuration of products cis-3aa and trans-3aa′ were further confirmed by single crystal X-ray diffraction analysis.12
1) compared to those of AgNTf2 and AgBF4 (Table 1, entries 17–19). Further attempts to modify the pyridine-based phosphine ligands L10–L12 failed to improve the yield and diastereoselectivity (Table 1, entries 17–19). The solvent screening of, e.g., toluene and DCE could not give better results (Table 1, entries 23, 24 and Table S1 in the ESI†). The introduction of diazo compound 1a in one portion led to a lower yield (Table 1, entry 25). A series of other catalysts, such as Cu(OTf)2, AgOTf, and (C6F5)3B, which are commonly used in the transformation of diazo compounds, was also tested, and they showed very low catalytic activity and selectivity in this case (Table S1 in the ESI†). The structure and the relative configuration of products cis-3aa and trans-3aa′ were further confirmed by single crystal X-ray diffraction analysis.12
| Entry | Cat. (5 mol%) | Solvent | Yieldb (%) |
|---|---|---|---|
| 3aa/3aa′/4aa | |||
| a A solution of 1a (0.4 mmol) in 1 mL of CH2Cl2 was introduced to a mixture of 2a (0.6 mmol) and catalyst (5 mol%) in solvent (5 mL) by syringe in 20 min, and the reaction mixture stirred for 6 h. b NMR yield. c The solution of 1a was added directly. Johnphos = 2-(di-tert-butylphos-phino)biphenyl; IPr = 1,3-bis(2,6-di-ipropyl-phenyl)imidazol-2-ylidene. | |||
| 1 | (2,4-tBu2C6H3O)3PAuCl/AgNTf2 | DCM | Messy |
| 2 | PPh3AuCl/AgNTf2 | DCM | Messy |
| 3 | IPrAuCl/AgNTf2 | DCM | Trace |
| 4 | JohnPhosAuCl/AgNTf2 | DCM | 51/24/6 |
| 5 | t Bu3PAuCl/AgNTf2 | DCM | 59/16/5 |
| 6 | Ph2MePAuCl/AgNTf2 | DCM | 56/16/8 |
| 7 | L1AuCl/AgNTf2 | DCM | Trace |
| 10 | L2AuCl/AgNTf2 | DCM | 15/45/5 |
| 11 | L3AuCl/AgNTf2 | DCM | 48/24/6 |
| 12 | L4AuCl/AgNTf2 | DCM | 39/17/13 |
| 13 | L5AuCl/AgNTf2 | DCM | Trace |
| 14 | L6AuCl/AgNTf2 | DCM | 32/8/12 |
| 15 | L7AuCl/AgNTf2 | DCM | 46/24/9 |
| 16 | L8AuCl/AgNTf2 | DCM | 59/12/5 |
| 17 | L9AuCl/AgNTf2 | DCM | 77/17/2 |
| 18 | L9AuCl/AgBF4 | DCM | 36/18/9 |
| 19 | L9AuCl/NaBAr F | DCM | 85/11/2 |
| 20 | L10AuCl/NaBArF | DCM | 79/11/3 |
| 21 | L11AuCl/NaBArF | DCM | 55/16/3 |
| 22 | L12AuCl/NaBArF | DCM | 80/17/2 |
| 23 | L9AuCl/NaBArF | DCE | 61/10/0 |
| 24 | L9AuCl/NaBArF | Toluene | 45/14/3 |
| 25c | L9AuCl/NaBArF | DCM | 59/8/— |
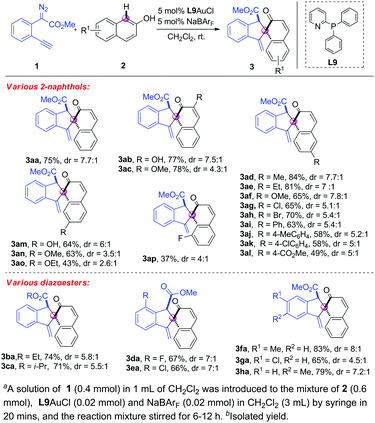
With the optimal reaction conditions in hand (Table 1, entry 19), we next investigated the substrate scope of the 2-naphthols 1. As shown in Scheme 2, a diverse range of 2-naphthols were suitable substrates for this tandem C–H bond functionalization/5-exo-dig carbocyclisation reaction, affording the corresponding spirocyclic products in moderate to good yields with excellent chemo- and site-selectivity and good diastereo-selectivity. Various commonly encountered functional groups such as hydroxyl, alkoxyl, alkyl, chloro, bromo and aryl at C3 and C6-position were well tolerated (3aa–3ak). Gratifyingly, 2-naphthol 2l with the strong electron-withdrawing group also reacted with alkynyl diazoester 1a smoothly to give the corresponding spirocyclic product 3al in 49% yield and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The lower yield should be attributed to the weaker nucleophile of 2l with the ester group that slows down the C–H bond functionalization and the sequential cyclisation. C7-substituted substrates 2m–2o were compatible under the reaction conditions, giving the spirocyclic 3am–3ao in good yields with good dr ratios. It was noteworthy that the reaction of C8-substituted 2-naphthol showed lower reactivity, probably due to the bulky allylic 1,3-strain (3ap). Subsequently, a variety of o-alkynylaryl-α-diazoesters 1b–1h were prepared and tested. All the reactions of 2a with 1b–1h, which were equipped with various ester groups and phenyl rings, worked smoothly, delivering the corresponding dearomatization products 3ba–3ha in good yields with good diastereoselectivity (Scheme 3). It was noteworthy that all the [4+1] spiroannulations were highly chemoselective and ortho-selective.
1 dr. The lower yield should be attributed to the weaker nucleophile of 2l with the ester group that slows down the C–H bond functionalization and the sequential cyclisation. C7-substituted substrates 2m–2o were compatible under the reaction conditions, giving the spirocyclic 3am–3ao in good yields with good dr ratios. It was noteworthy that the reaction of C8-substituted 2-naphthol showed lower reactivity, probably due to the bulky allylic 1,3-strain (3ap). Subsequently, a variety of o-alkynylaryl-α-diazoesters 1b–1h were prepared and tested. All the reactions of 2a with 1b–1h, which were equipped with various ester groups and phenyl rings, worked smoothly, delivering the corresponding dearomatization products 3ba–3ha in good yields with good diastereoselectivity (Scheme 3). It was noteworthy that all the [4+1] spiroannulations were highly chemoselective and ortho-selective.
 | (1) |
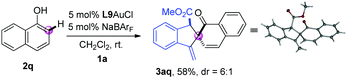
Next, the dearomatization of 1-naphthol was also investigated. The combination of 1-naphthol 2q and o-alkynylaryl-α-diazoester 1a underwent tandem ortho-selective C–H bond functionalization/dearomative cyclisation reaction under the standard conditions, giving the corresponding spirocycle product 3aq in 58% yield with 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (eqn (1)). The structure of the product 3aq was further confirmed by single crystal X-ray diffraction analysis.12
1 dr (eqn (1)). The structure of the product 3aq was further confirmed by single crystal X-ray diffraction analysis.12
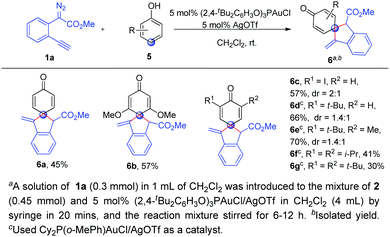
Finally, we wondered whether phenols were applicable to the present spiroannulation, which was more challenging because the energy barrier of dearomatization of phenols is significantly higher than that of naphthols.13 Unfortunately, the reaction of phenol 5a with alkynyl diazoester 1a gave a complicated mixture under the standard conditions. From our previous work, we knew that the gold catalysts for C–H bond functionalization of phenols and naphthols were very different. After switching the ligand, to our delight, (2,4-tBu2C6H3O)3PAuCl/AgOTf could enable the reaction of phenol 5a with 1a to give the corresponding dearomatization product 6a in moderate yield. This high para-selectivity was consistent with our previous studies.10e When the phenols 5b–5g with various substituents such as alkyls, alkoxyl etc. were used, the corresponding para C–H bond functionalization/dearomatization products 6b–6g were isolated in moderate to good yields (Scheme 3). Unfortunately, the reaction of p-methyl phenol with 1a cannot give the ortho-spiroannulation product but afforded the O–H insertion product.
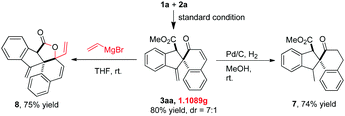
It should be noted that this gold(I)-catalyzed cascade C–H functionalization/dearomatization of naphthols with o-alkynylaryl-α-diazoesters was easy to scale-up. A gram-scale reaction of 5 mmol of 1a and 2a was carried out under standard conditions, furnishing 1.1089 g of 3aa in 70% isolated yield and 3aa′ in 10% isolated yield (Scheme 4). To demonstrate the synthetic value of this protocol further, transformations of 3aa were performed (Scheme 4). The reduction of the exocyclic double bond of 3aa would produce the corresponding product 7 in 74% yield. The reaction of 3aa with vinyl magnesium bromide afforded the multi-fused lactone 8 in 75% yield via the tandem 1,2-addition/lactonization.
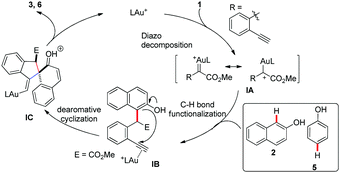
Based on the above results and previously reported work, a proposed mechanistic pathway accounting for this transformation is illustrated in Scheme 5. The gold(I) carbene intermediate IA, which was formed from o-alkynylaryl α-diazoesters 1 with the gold catalyst, would react with the nucleophilic naphthols 2 or phenols 5 to afford ortho- or para-selective C–H bond functionalization product IB. The following 5-exo-dig carbocyclization of naphthols or phenols onto the terminal alkynes activated by the gold catalyst afforded the alkenyl gold intermediate IC. Finally, IC underwent the following deprotonation and protodeauration to produce the target spiroannulation products 3 or 6.
To conclude, we have developed a conceptually simple, but highly unusual protocol for achieving dearomatization of readily available phenols and naphthols. Upon treatment with the gold complex, highly chemo- and site-selective C–H bond functionalization of phenols and naphthols with o-alkynylaryl α-diazoesters occurs, delivering the alkynyl phenol derivatives, which can undergo the following carbocyclisation dearomatization reaction. This protocol provides a straightforward access to diverse highly complex three-dimensional spirocyclic molecules in good to excellent yields with high chemo- and regio-selectivity and good diastereo-selectivity. Moreover, this work will broaden the application of diazo compounds in organic synthesis and open a new door for the design of dearomatization of arenes.
We are grateful to the NSFC (No. 21971066 and 21772042) and the STCSM (18JC1412300) for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- Synthetic and Natural Phenols, ed. J. H. P. Tyman, Elsevier, 1996 Search PubMed.
- For selected books and reviews, see: reviews: (a) Asymmetric Dearomatization Reactions, ed. S.-L. You, Wiley-VCH, Weinheim, Germany, 2016 Search PubMed; (b) A. R. Pape, K. P. Kaliappan and E. P. Kündig, Chem. Rev., 2000, 100, 2917–2940 CrossRef CAS PubMed; (c) S. P. Roche and J. Porco, Jr., Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed; (d) C. Zheng and S.-L. You, Chem, 2016, 1, 830 CrossRef CAS.
- For selected reviews of dearomatization of phenols and naphthols, see: (a) H. Wang and X. Luan, Org. Biomol. Chem., 2016, 14, 9451–9455 RSC; (b) W.-T. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570–1580 RSC; (c) W. Sun, G. Li, L. Hong and R. Wang, Org. Biomol. Chem., 2016, 14, 2164–2176 RSC.
- For selected examples of intramolecular dearomatizaion of phenols and naphthols to construct spirocycles, see: (a) T. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno and Y. Hamada, Org. Lett., 2010, 12, 5020–5023 CrossRef CAS PubMed; (b) S. Rousseaux, J. García-Fortanet, M. A. Del Aguila Sanchez and S. L. Buchwald, J. Am. Chem. Soc., 2011, 133, 9282–9285 CrossRef CAS PubMed; (c) Q.-F. Wu, W.-B. Liu, C.-X. Zhuo, Z.-Q. Rong, K.-Y. Ye and S.-L. You, Angew. Chem., Int. Ed., 2011, 50, 4455–4458 CrossRef CAS PubMed; (d) R.-Q. Xu, Q. Gu, W.-T. Wu, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2014, 136, 15469–15472 CrossRef CAS PubMed; (e) H. Nakayama, S. Harada, M. Kono and T. Nemoto, J. Am. Chem. Soc., 2017, 139, 10188–10191 CrossRef CAS PubMed.
- For intermolecular spiroannulation, see: (a) L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang and X. Luan, J. Am. Chem. Soc., 2015, 137, 4876–4879 CrossRef CAS; (b) H. Zheng, L. Bai, J. Liu, J. Nan, Z. Zuo, L. Yang, Y. Wang and X. Luan, Chem. Commun., 2015, 51, 3061–3064 RSC; (c) Z. Zuo, J. Wang, J. Liu, Y. Wang and X. Luan, Angew. Chem., Int. Ed., 2020, 59, 653–657 CrossRef CAS PubMed.
- For selected recent reviews on synthesis of spirocycles, see: (a) P.-W. Xu, J.-S. Yu, C. Chen, Z.-Y. Cao, F. Zhou and J. Zhou, ACS Catal., 2019, 9, 1820–1882 CrossRef CAS; (b) E. M. Carreira and T. C. Fessard, Chem. Rev., 2014, 114, 8257–8322 CrossRef CAS PubMed.
- (a) C. Cheng and J. F. Hartwig, Chem. Rev., 2015, 115, 8946 CrossRef CAS PubMed; (b) G. Song and X. Li, Acc. Chem. Res., 2015, 48, 1007 CrossRef CAS; (c) K. Shin, H. Kim and S. Chang, Acc. Chem. Res., 2015, 48, 1040 CrossRef CAS; (d) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Q. Yu, Chem. Rev., 2017, 117, 8754–8786 CrossRef CAS PubMed.
- For [3+2] dearomatization of 2-naphthol derivatives, see: (a) J. Zheng, S.-B. Wang, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2015, 137, 4880–4883 CrossRef CAS; (b) C. Zheng, J. Zheng and S.-L. You, ACS Catal., 2016, 6, 262–271 CrossRef CAS; (c) G. Duarah, P. P. Kaishap, B. Sarma and S. Gogoi, Chem. – Eur. J., 2018, 24, 10196–10200 CrossRef CAS; (d) I. Khan, S. R. Chidipudiab and H. W. Lam, Chem. Commun., 2015, 51, 2613–2616 RSC; (e) J. Nan, Z. Zuo, L. Luo, L. Bai, H. Zheng, Y. Yuan, J. Liu, X. Luan and Y. Wang, J. Am. Chem. Soc., 2013, 135, 17306–17309 CrossRef CAS; (f) L. Han, H. Wang and X. Luan, Org. Chem. Front., 2018, 5, 2453–2457 RSC . For [3+2] dearomatization of phenol derivatives, see: ; (g) A. Seoane, N. Casanova, N. Quiñones, J. L. Mascareñas and M. Gulías, J. Am. Chem. Soc., 2014, 136, 7607–7610 CrossRef CAS PubMed; (h) P.-P. Lin, X.-L. Han, G.-H. Ye, J.-L. Li, Q. Li and H. Wang, J. Org. Chem., 2019, 84, 12966–12974 CrossRef CAS; (i) S. Kujawa, D. Best, D. J. Burns and H. W. Lam, Chem. – Eur. J, 2014, 20, 8599–8602 CrossRef CAS PubMed . For [2+2+1] dearomatization of 2-naphthol, see: ; (j) S. Gu, L. Luo, J. Liu, L. Bai, H. Zheng, Y. Wang and X. Luan, Org. Lett., 2014, 16, 6132–6135 CrossRef CAS PubMed.
- For reviews on gold-catalyzed transformation of diazo compounds, see: (a) L. Liu and J. Zhang, Chem. Soc. Rev., 2016, 45, 506 RSC; (b) F. Wei, C. Song, Y. Ma, L. Zhou, C.-H. Tung and Z. Xu, Sci. Bull., 2015, 60, 1479 CrossRef CAS; (c) M. R. Fructos, M. M. Díaz-Requejo and P. J. Pérez, Chem. Commun., 2016, 52, 7326 RSC; (d) B. Ma, L. Liu and J. Zhang, Asian J. Org. Chem., 2018, 7, 2015–2025 CrossRef CAS.
- For reviews, see: (a) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704 CrossRef CAS; (b) M. M. Díaz-Requejo and P. J. Pérez, Chem. Rev., 2008, 108, 3379 CrossRef; (c) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981 CrossRef CAS; (d) L. Liu and J. Zhang, Chin. J. Org. Chem., 2017, 37, 1117–1126 CrossRef CAS . For examples of our group, see: ; (e) Z. Yu, B. Ma, M. Chen, H.-H. Wu, L. Liu and J. Zhang, J. Am. Chem. Soc., 2014, 136, 6904 CrossRef CAS PubMed; (f) B. Ma, Z. Chu, B. Huang, Z. Liu, L. Liu and J. Zhang, Angew. Chem., Int. Ed., 2017, 56, 2749 CrossRef CAS PubMed; (g) Y. Liu, Z. Yu, J. Z. Zhang, L. Liu, F. Xia and J. Zhang, Chem. Sci., 2016, 7, 1988 RSC; (h) Z. Yu, Y. Li, P. Zhang, L. Liu and J. Zhang, Chem. Sci., 2019, 10, 6553–6559 RSC; (i) Z. Yu, H. Qiu, L. Liu and J. Zhang, Chem. Commun., 2016, 52, 2257 RSC; (j) B. Ma, Z. Wu, B. Huang, L. Liu and J. Zhang, Chem. Commun., 2016, 52, 9351 RSC; (k) B. Ma, J. Wu, L. Liu and J. Zhang, Chem. Commun., 2017, 53, 10164–10167 RSC.
- (a) W.-T. Wu, R.-Q. Xu, L. Zhang and S.-L. You, Chem. Sci., 2016, 7, 3427 RSC . For reviews, see: ; (b) W.-T. Wu, L. Zhang and S.-L. You, Acta Chim. Sin., 2017, 75, 419–438 CrossRef CAS.
- CCDC 1915415 (3aa), 1915417 (3aa′), 1915418 (3aq)†.
- T. Oguma and T. Katsuki, J. Am. Chem. Soc., 2012, 134, 20017–20020 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1915415, 1915417 and 1915418. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc01118e |
| This journal is © The Royal Society of Chemistry 2020 |