Access to 4-substituted isothiazoles through three-component cascade annulation and their application in C–H activation†
Abstract
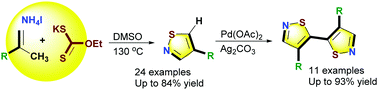
The use of potassium ethyl xanthate (EtOCS2k) as a sulfur atom donor enabled the transition-metal-free [3 + 1 + 1] cascade annulation of isopropene derivatives with NH4I in DMSO/H2O, affording various 4-substituted isothiazoles in moderate to good yields with good functional group compatibility. Furthermore, Pd and Ag-catalyzed C5–H-selective direct oxidation dimerization of 4-substituted isothiazoles afforded 5,5′-bisisothiazoles. This series of reactions included the formation of new C–S, C–N, N–S, and C–C bonds via cascade addition, annulation, and direct C–H activation under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...