Enantioconvergent alkylation of ketones with racemic secondary alcohols via hydrogen borrowing catalysis†
Abstract
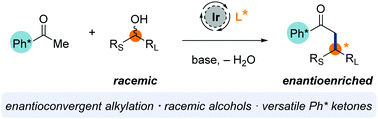
An enantioconvergent method for the alkylation of o-disubstituted aryl ketones with racemic secondary alcohols is described. This process is mediated by a commercially available iridium catalyst and proceeds via hydrogen borrowing catalysis. The highly enantioenriched β-substituted ketone products were readily cleaved to a wide range of functional groups via retro-Friedel–Crafts acylation.


 Please wait while we load your content...
Please wait while we load your content...