 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chirality sensing of terpenes, steroids, amino acids, peptides and drugs with acyclic cucurbit[n]urils and molecular tweezers†
Amrutha
Prabodh
 a,
Daniel
Bauer
b,
Stefan
Kubik
a,
Daniel
Bauer
b,
Stefan
Kubik
 b,
Philipp
Rebmann
c,
Frank Gerritt
Klärner
c,
Thomas
Schrader
b,
Philipp
Rebmann
c,
Frank Gerritt
Klärner
c,
Thomas
Schrader
 c,
Lorenzo
Delarue Bizzini
c,
Lorenzo
Delarue Bizzini
 d,
Marcel
Mayor
d,
Marcel
Mayor
 ad and
Frank
Biedermann
ad and
Frank
Biedermann
 *a
*a
aKarlsruhe Institute of Technology (KIT), Institute of Nanotechnology (INT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany. E-mail: frank.biedermann@kit.edu
bTechnische Universität Kaiserslautern, Fachbereich Chemie – Organische Chemie, Erwin-Schrödinger-Straße, 67663 Kaiserslautern, Germany
cUniversity of Duisburg-Essen, Faculty of Chemistry, Universitätsstr. 7, 45117 Essen, Germany
dUniversity of Basel, Department of Chemistry, St. Johanns-Ring 19, 4056 Basel, Switzerland
First published on 25th February 2020
Abstract
Achiral chromophoric hosts, i.e. acyclic cucurbit[n]urils and molecular tweezers, were found to respond with characteristic Circular Dichroism (CD) spectra to the presence of micromolar concentrations of chiral hydrocarbons, terpenes, steroids, amino acids and their derivates, and drugs in water. In favourable cases, this allows for analyte identification or for reaction monitoring.
Chirality is an inherent property of many compounds of biological origin such as amino acids, peptides and proteins, but also widely present in synthetic molecules such as drugs. Chiral substances can be characterized by chiroptical spectroscopic methods, e.g. Electronic Circular Dichroism (ECD), which are usually more “information-rich” than their non-chiral spectroscopic counterparts.1–7 However, most bioactive small molecules such as amino acids are non-chromophoric or absorb below 280 nm of the electromagnetic spectrum. Besides, small molecules, are typically conformationally flexible, causing an averaged ECD signal.1 Thus, ECD spectroscopy is of limited use for detecting or identification of metabolites, hormones and peptides. In recent years, molecular recognition-based approaches were introduced for chirality sensing of chromophoric small molecules by ECD spectroscopy.7–12
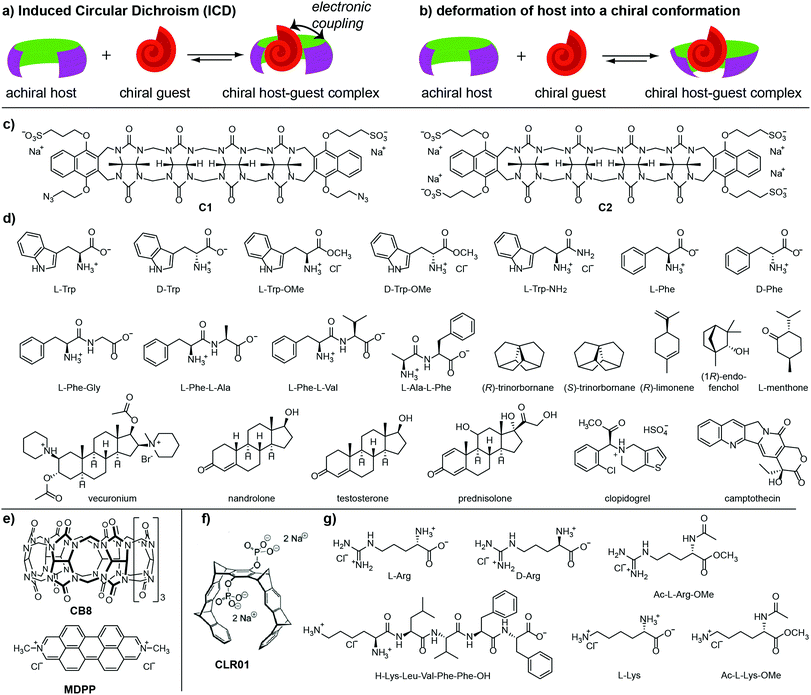
The spatial proximity and controlled orientation between a chiral analyte and an achiral chromophoric host in their host–guest complex can result in an electronic coupling, giving rise to induced Circular Dichroism (ICD), see Fig. 1a.4,13–17 In favourable cases, analyte-specific “ICD fingerprints” occur, which can be utilised for analyte identification and differentiation.5,17–19 Hydrogen bonding,2,20–22 metal coordination,6,23–26 or dynamic covalent bonds3,27–32 were frequently used as directional bonding motifs, leading to well-defined binding conformations.21 For the detection of compounds in aqueous media, only a few chirality-based chemosensors are available15,17,33–35 because directional, polar non-covalent interactions are screened by the solvent. Furthermore, the hydrophobic effect as the most important driving force for binding lacks directionality.36–38 Concave hosts can provide both strong hydrophobic binding forces and restrict the number of host–guest conformations. For instance, endo-functionalized molecular tubes can selectively recognize chiral epoxides in water, giving rise to strong ICD signals.15 Similarly, the noncovalent chemosensing ensembles composed of the macrocycle cucurbit[8]uril (CB8) and dicationic dyes are suitable for chirality sensing of aromatic compounds.17
We wondered if the concept of “chirality transfer” to a chromophoric achiral and concave host can be extended to acyclic cucurbit[n]urils39–41 and molecular tweezers,42–47 which both display sizeable binding constants for small bioactive molecules and engulf their guests inside their concave cavity. Acyclic cucurbit[n]urils were shown to bind a broad range of bioactive molecules, e.g. drugs, hormones and nucleotides.39–41 Molecular tweezers are more selective binders, for instance, for lysine, arginine and their derivatives.43–45 They also selectively recognize peptides and proteins with sterically accessible Lys and Arg residues. In this contribution, we systematically investigate Circular Dichroism detected chirality sensing with acyclic cucurbit[n]urils or molecular tweezers as hosts for chiral guests in water. The chemical structures of the hosts and analytes tested are shown in Fig. 1(c–g).
The two acyclic CBn (C1 and C2) (Fig. 1c) were prepared via a stepwise oligomerization procedure39,48 and differ in their charge, i.e. C1 is a dianion and C2 a tetraanion. In order to assess the utility of the acyclic CBn for chirality sensing, the chiral aromatic amino acids L-Phe and L-Trp were added to aqueous solutions of the host C1 and the Circular Dichroism spectra were recorded, Fig. 2a and Fig. S2a (ESI†). A strong positive CD band at 292 nm and a weaker one at 326 nm was observed for the supramolecular complexes of C1 with either L-Phe or L-Trp.
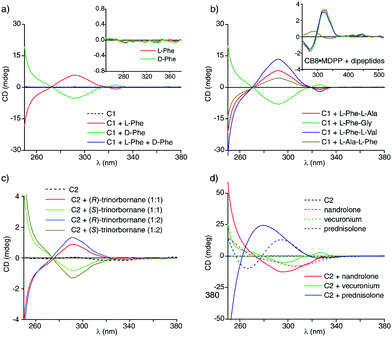 | ||
| Fig. 2 (a) ECD spectra in water for host C1 (100 μM) in the presence of L-Phe (100 μM), D-Phe (100 μM) and for a racemic mixture composed of 100 μM L-Phe and 100 μM D-Phe. The inset shows the ECD spectra of the guests alone. (b) ECD spectra in water for host C1 (100 μM) in the presence of Phe-containing dipeptides (each at 100 μM). The inset shows the ECD spectra of the same guests (each at 50 μM) in the presence of the CB8·MDPP (20 μM) chemosensing ensemble. The guest alone showed no significant CD signals, see the ESI.† (c) ECD spectra in water for host C2 (100 μM) in the presence of (R)- or (S)-trinorbornane (99 μM) as well as excess of the analyte (197 μM) (with ≤1.2 vol% ACN). (d) ECD spectra in water for the steroids nandrolone, vecuronium and prednisolone (each at 100 μM) in the presence and absence of the host C2 (100 μM). | ||
Their enantiomers D-Phe and D-Trp gave the expected mirrored CD spectra; supramolecular host–guest complexation with acyclic CBn can thus be utilized for monitoring of optical purity. As an example, base-catalysed racemization of L-Phe and the peptide L-Phe-Gly in organic solvents (DMF, ethylene glycol) and water was monitored by CD spectroscopy via adding aliquots of the reaction mixture to the aqueous host solution. In accordance with the literature,49 it was found that water suppressed racemization that is occurring in DMF at increased temperature (Fig. S8, ESI†). The chemosensor-based monitoring approach is faster than the established chromatography-based method,49 and thus allows for screening of reaction conditions.
The complexation of phenylalanine and tryptophan derivatives by host C1 led to a completely different type of ECD spectra than observed for Phe/Trp-species bound by the CB8·MDPP chemosensing ensemble: for acyclic CBn·guest complexes, the ECD band position and shapes (e.g. a stronger band at 292 nm and a weaker one at 326 nm) coincide with the absorbance band maxima of the free host (Fig. S3a and S4a, ESI†). Furthermore, ECD spectra of C1 or C2 complexes with different chiral guests do not display unique CD-spectral bands but rather differ only in the signal magnitude. Conversely, the ECD spectra of the CB8·MDPP chemosensing ensemble (Fig. 1e) displayed indicative spectral fingerprints for different phenylalanine and tryptophan derivatives (Fig. S3c and S4c, ESI†). Moreover, the band shape in the ECD spectrum clearly differed from that in the absorbance spectrum of the CB8·MDPP chemosensing ensemble. Two different mechanisms may therefore be at work: (i) for acyclic CBn·guest complexes, the host deforms into a chiral conformation upon binding the chiral analyte (Fig. 1b). Different chiral analytes may cause a different degree of host deformation, and thus are characterized by different signal magnitudes in the ECD spectra. (ii) For complexes of aromatic chiral guests with CB8·MDPP,17 there is a major contribution of a transition dipole coupling between the dicationic MDPP chromophore and the aryl moiety of the guest, leading to guest-indicative induced circular dichroism (ICD) bands. Similarly, changing the chromophore to MDAP17 leads to completely new ICD bands and trends for the same series of chiral guests (Fig. S5, ESI†) as expected for an ICD effect. Because both CB8 and MDPP (or MDAP) are rather rigid, there is likely no significant contribution of chiral deformation of the host.
As a consequence of the different CD signal generation mechanisms for C1/C2 and CB8·MDPP, they can be used complementarily. For example, it is possible to distinguish the dipeptides L-Phe-Gly, L-Phe-L-Ala, L-Phe-L-Val and L-Ala-L-Phe from each other through binding with C1 (Fig. 2b) while with the CB8·MDPP chemosensing ensemble only L-Ala-L-Phe can be differentiated from the other peptides by CD spectroscopy (Fig. 2b, inset). In favourable cases, also simple mixtures of peptides, e.g.L-Phe-Gly and L-Phe-L-Val can be deconvoluted, e.g. by using C1 as the host (Fig. S6, ESI†) while the CB8·MDPP chemosensing ensemble is particularly useful for analysing mixtures of Phe- and Trp-species (Fig. S7, ESI†).
Because acyclic CBn bind a wide range of hydrophobic molecules, and because no ICD signal generation is required for acyclic CBn, these hosts can be used for chirality sensing of analyte classes that are beyond the scope of previously reported chemosensing ensembles. For instance, complexation of the chiral bridged-alkane trinorbornane50,51 by host C2 gave rise to clear CD signals despite the completely non-chromophoric nature of the hydrocarbon guest (Fig. 2c). Likewise, terpenes, limonene and fenchol as well as the steroidal drug vecuronium do not show ECD signals above 250 nm. However, in the presence of host C1, both (R)-limonene and (1R)-endo-(+)-fenchol (Fig. S10a, ESI†) as well as vecuronium (Fig. S12a, ESI†) clearly display bands in the ECD spectrum up to 340 nm, which can be attributed to the chiral induction upon supramolecular complex formation (Fig. 1b). C1 and C2 also bind efficiently other steroids such as nandrolone and prednisolone in water; those chromophoric steroids possess CD signals on their own but binding to C1 or C2 causes characteristic shifts and increases the signal intensities in the CD spectra (Fig. 2d). Besides, the different host variants C1 and C2 gave rise to different induced CD spectra with these steroids which may be useful for pattern-recognition52 based steroid identification (Fig. S12, ESI†). In principle, it is also possible to deconvolute steroid mixtures using the host–guest binding-induced circular dichroism signals (Fig. S17, ESI†). Likewise, strong CD signals were observed when the water-insoluble chiral drugs testosterone, camptothecin and clopidogrel were solubilized in water through binding with acyclic CBn,39i.e. by both C1 and C2 (Fig. S16a and b, ESI†) (Control experiments where the drugs are solubilized in ethanol are shown in Fig. S15c, ESI† and gave comparable results). Again, binding of the chiral chromophoric guests by the achiral chromophoric host causes characteristic changes in the ECD spectrum.
Unlike acyclic CBn, molecular tweezer CLR01 (Fig. 1f) is a rigid host and thus it is unlikely that upon inclusion of chiral guests a substantial chiral twist of the host structure occurs.45
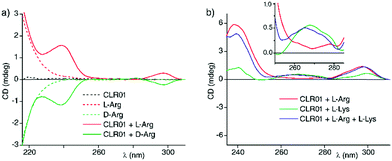
Nevertheless, we found that ECD spectra for the complexes of molecular tweezer CLR01 with several Lys and Arg derivatives in water show distinguishable chiral ECD spectral fingerprints (Fig. 3a and Fig. S19–S22, ESI†). We tentatively explain the ICD through coupling of the transition dipole of the host with that of the chromophores of the Lys/Arg derivatives (e.g. the amide groups). In analogy to the aforementioned examples, an ECD-based sensing protocol can in principle be established with host CLR01 to deconvolute mixtures of Lys- and Arg-derivatives (Fig. 3b).
Surprisingly, the ICD for arginine is much larger than that for Lysine, although CLR01 binds Lys tighter.42 This may be used to distinguish between basic residues on structurally complex peptides and proteins, which often contain multiple lysines and arginines. To date, structural information about the preferred tweezer binding sites on peptides and proteins must be derived from 2D/3D NMR spectra and crystal structures.45
In summary, we have shown that the formation of chiral supramolecular host–guest complexes self-assembled from achiral, chromophoric hosts and chiral (non-)chromophoric small molecule guests can give information-rich Circular Dichroism spectra in aqueous media with potential utility for chirality sensing, analyte identification and reaction monitoring applications. Our finding suggests two tentative host design principles for chirality sensing: (1) if a host should provide analyte-indicative ICD fingerprints, then the use of rigid host structures is recommended. (2) General binders for chiral guests should possess a flexible and adaptable host structure that adopts a chiral, twisted conformation upon binding of the chiral guests. Such hosts are then also applicable for chirality sensing of non-chromophoric guests.
This work was financially supported through grants by the German Academic Exchange Service (DAAD) (A. P.), DFG (F. B.), the DFG-funded collaborative research centre CRC 1093 (T. S.), and a contract (E/U2AD/GD008/GF565) with the German Armed Forces (S. K.)
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- N. Berova, L. Di Bari and G. Pescitelli, Chem. Soc. Rev., 2007, 36, 914–931 RSC
.
- H. Gholami, M. Anyika, J. Zhang, C. Vasileiou and B. Borhan, Chem. – Eur. J., 2016, 22, 9235–9239 CrossRef CAS PubMed
.
- C. Wolf and K. W. Bentley, Chem. Soc. Rev., 2013, 42, 5408–5424 RSC
.
- P. Metola, E. V. Anslyn, T. D. James and S. D. Bull, Chem. Sci., 2012, 3, 156–161 RSC
.
- K. W. Bentley, Y. G. Nam, J. M. Murphy and C. Wolf, J. Am. Chem. Soc., 2013, 135, 18052–18055 CrossRef CAS PubMed
.
- G. Proni, G. Pescitelli, X. Huang, K. Nakanishi and N. Berova, J. Am. Chem. Soc., 2003, 125, 12914–12927 CrossRef CAS PubMed
.
- L. You, J. S. Berman and E. V. Anslyn, Nat. Chem., 2011, 3, 943–948 CrossRef CAS PubMed
.
- L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840–7892 CrossRef CAS PubMed
.
- G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1–73 CrossRef CAS PubMed
.
- Z. Chen, Q. Wang, X. Wu, Z. Li and Y. B. Jiang, Chem. Soc. Rev., 2015, 44, 4249–4263 RSC
.
- L. J. Chen, H. B. Yang and M. Shionoya, Chem. Soc. Rev., 2017, 46, 2555–2576 RSC
.
- S. Tashiro, Y. Ogura, S. Tsuboyama, K. Tsuboyama and M. Shionoya, Inorg. Chem., 2011, 50, 4–6 CrossRef CAS PubMed
.
- N. Berova, G. Pescitelli, A. G. Petrovic and G. Proni, Chem. Commun., 2009, 5958–5980 RSC
.
- F. A. Scaramuzzo, G. Licini and C. Zonta, Chem. – Eur. J., 2013, 19, 16809–16813 CrossRef CAS PubMed
.
- L. L. Wang, Z. Chen, W. E. Liu, H. Ke, S. H. Wang and W. Jiang, J. Am. Chem. Soc., 2017, 139, 8436–8439 CrossRef CAS PubMed
.
- M. Sapotta, P. Spenst, C. R. Saha-Möller and F. Würthner, Org. Chem. Front., 2019, 6, 892–899 RSC
.
- F. Biedermann and W. M. Nau, Angew. Chem., Int. Ed., 2014, 53, 5694–5699 CrossRef CAS PubMed
.
- K. W. Bentley and C. Wolf, J. Org. Chem., 2014, 79, 6517–6531 CrossRef CAS PubMed
.
- H. Kim, S. M. So, C. P. Yen, E. Vinhato, A. J. Lough, J. I. Hong, H. J. Kim and J. Chin, Angew. Chem., Int. Ed., 2008, 47, 8657–8660 CrossRef CAS PubMed
.
- M. Inouye, M. Waki and H. Abe, J. Am. Chem. Soc., 2004, 126, 2022–2027 CrossRef CAS PubMed
.
- M. Anyika, H. Gholami, K. D. Ashtekar, R. Acho and B. Borhan, J. Am. Chem. Soc., 2014, 136, 550–553 CrossRef CAS PubMed
.
- M. J. Kim, Y. R. Choi, H. G. Jeon, P. Kang, M. G. Choi and K. S. Jeong, Chem. Commun., 2013, 49, 11412–11414 RSC
.
- K. W. Bentley, P. Zhang and C. Wolf, Sci. Adv., 2016, 2, e1501162 CrossRef PubMed
.
- X. Li, C. E. Burrell, R. J. Staples and B. Borhan, J. Am. Chem. Soc., 2012, 134, 9026–9029 CrossRef CAS PubMed
.
- S. J. Wezenberg, G. Salassa, E. C. Escudero-Adan, J. Benet-Buchholz and A. W. Kleij, Angew. Chem., Int. Ed., 2011, 50, 713–716 CrossRef CAS PubMed
.
- H. Tsukube and S. Shinoda, Chem. Rev., 2002, 102, 2389–2403 CrossRef CAS PubMed
.
- C. Ni, D. Zha, H. Ye, Y. Hai, Y. Zhou, E. V. Anslyn and L. You, Angew. Chem., Int. Ed., 2018, 57, 1300–1305 CrossRef CAS PubMed
.
- S. L. Pilicer, P. R. Bakhshi, K. W. Bentley and C. Wolf, J. Am. Chem. Soc., 2017, 139, 1758–1761 CrossRef CAS PubMed
.
- C. Y. Lin, M. W. Giuliano, B. D. Ellis, S. J. Miller and E. V. Anslyn, Chem. Sci., 2016, 7, 4085–4090 RSC
.
- Z. A. De Los Santos and C. Wolf, J. Am. Chem. Soc., 2016, 138, 13517–13520 CrossRef CAS PubMed
.
- X. X. Chen, Y. B. Jiang and E. V. Anslyn, Chem. Commun., 2016, 52, 12669–12671 RSC
.
- J. B. Xiong, H. T. Feng, J. P. Sun, W. Z. Xie, D. Yang, M. Liu and Y. S. Zheng, J. Am. Chem. Soc., 2016, 138, 11469–11472 CrossRef CAS PubMed
.
- T. Morozumi and S. Shinkai, J. Chem. Soc., Chem. Commun., 1994, 1219–1220 RSC
.
- L. Vial, M. Dumartin, M. Donnier-Marechal, F. Perret, J. P. Francoia and J. Leclaire, Chem. Commun., 2016, 52, 14219–14221 RSC
.
- H. Goto, Y. Furusho and E. Yashima, Chem. Commun., 2009, 1650–1652 RSC
.
- F. Biedermann, W. M. Nau and H. J. Schneider, Angew. Chem., Int. Ed., 2014, 53, 11158–11171 CrossRef CAS PubMed
.
- F. Biedermann and H. J. Schneider, Chem. Rev., 2016, 116, 5216–5300 CrossRef CAS PubMed
.
- S. He, F. Biedermann, N. Vankova, L. Zhechkov, T. Heine, R. E. Hoffman, A. De Simone, T. T. Duignan and W. M. Nau, Nat. Chem., 2018, 10, 1252–1257 CrossRef CAS PubMed
.
- D. Ma, G. Hettiarachchi, D. Nguyen, B. Zhang, J. B. Wittenberg, P. Y. Zavalij, V. Briken and L. Isaacs, Nat. Chem., 2012, 4, 503–510 CrossRef CAS PubMed
.
- T. Minami, N. A. Esipenko, B. Zhang, L. Isaacs and P. Anzenbacher, Jr., Chem. Commun., 2014, 50, 61–63 RSC
.
- S. A. Zebaze Ndendjio and L. Isaacs, Supramol. Chem., 2019, 31, 432–441 CrossRef CAS
.
- S. Dutt, C. Wilch, T. Gersthagen, P. Talbiersky, K. Bravo-Rodriguez, M. Hanni, E. Sanchez-Garcia, C. Ochsenfeld, F. G. Klaerner and T. Schrader, J. Org. Chem., 2013, 78, 6721–6734 CrossRef CAS PubMed
.
- T. Schrader, G. Bitan and F. G. Klaerner, Chem. Commun., 2016, 52, 11318–11334 RSC
.
- M. Fokkens, T. Schrader and F. G. Klaerner, J. Am. Chem. Soc., 2005, 127, 14415–14421 CrossRef CAS PubMed
.
- D. Bier, R. Rose, K. Bravo-Rodriguez, M. Bartel, J. M. Ramirez-Anguita, S. Dutt, C. Wilch, F. G. Klaerner, E. Sanchez-Garcia, T. Schrader and C. Ottmann, Nat. Chem., 2013, 5, 234–239 CrossRef CAS PubMed
.
- P. Talbiersky, F. Bastkowski, F. G. Klaerner and T. Schrader, J. Am. Chem. Soc., 2008, 130, 9824–9828 CrossRef CAS PubMed
.
- F. G. Klaerner and T. Schrader, Acc. Chem. Res., 2013, 46, 967–978 CrossRef CAS PubMed
.
- D. Bauer, B. Andrae, P. Gaß, D. Trenz, S. Becker and S. Kubik, Org. Chem. Front., 2019, 6, 1555–1560 RSC
.
- Y. Yokoyama, H. Hikawa and Y. Murakami, J. Chem. Soc., Perkin Trans. 1, 2001, 1431–1434 RSC
.
- L. Delarue Bizzini, T. Muntener, D. Haussinger, M. Neuburger and M. Mayor, Chem. Commun., 2017, 53, 11399–11402 RSC
.
- L. Delarue Bizzini, T. Bürgi and M. Mayor, Helv. Chim. Acta, 2020 DOI:10.1002/hlca.202000019
.
- A. I. Lazar, F. Biedermann, K. R. Mustafina, K. I. Assaf, A. Hennig and W. M. Nau, J. Am. Chem. Soc., 2016, 138, 13022–13029 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, experimental details, and fitting equations. See DOI: 10.1039/d0cc00707b |
| This journal is © The Royal Society of Chemistry 2020 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: