Open Access Article
Open Access ArticleCovalent polyester colouration by in situ chromophore creation†
Jason V.
Rowley
 a,
James
Exley
a,
Huayang
Yu
a,
James
Exley
a,
Huayang
Yu
 a,
Gregory S.
Mircale
b,
Adam S.
Hayward
c and
Paul D.
Thornton
a,
Gregory S.
Mircale
b,
Adam S.
Hayward
c and
Paul D.
Thornton
 *a
*a
aSchool of Chemistry, University of Leeds, LS2 9JT, UK. E-mail: p.d.thornton@leeds.ac.uk; Tel: +44 (0)113 3432935
bMiami Valley Laboratories, The Procter & Gamble Company, Cincinnati, Ohio, USA
cNewcastle Innovation Centre, The Procter & Gamble Company, Longbenton, Newcastle upon Tyne NE12 9TS, UK
First published on 30th April 2020
Abstract
The permanent colouration of a polyester by straightforward azo coupling is disclosed. Uniquely, the chromophore is created only upon successful polymer modification with a non-coloured molecule (in situ colouration), which confirms successful polymer adaptation and ensures that coloured waste is not produced. The method of colouration, which may feasibly be applied for the coloration of a wide-range of step-growth polyesters, yielded a polymer capable of preventing indigo deposition onto a range of fabrics, offering potential use within advanced detergent formulations.
Commodity polymers produced by step-growth polymerisations, for instance polyesters such as poly(ethylene terephthalate) (PET), are commonly coloured using disperse dyes owing to the lack of functionality that they present.1–3 Consequently, the dyeing of polyesters frequently necessitates the inclusion of dispersants, carriers, thickeners, and reducing agents as auxiliaries to achieve effective polymer colouration.4–6 Coloured wastewater is produced in the dyeing process that creates acute aesthetic, ecological and environmental problems.7,8 As disperse dye molecules do not covalently bind to the polymer substrate, long-term colouration is restricted by the disassociation of non-bound dye molecules over time.9
The covalent grafting of dye molecules provides permanent substrate colouration.10 Covalent colouration of step-growth polymers may proceed using dye molecules that form the terminal group of polymers,11 or by incorporating a difunctional dye within a step-growth poylmerisation.12 However, such methods use a pre-formed dye molecule that may be transferred to coloured effluent and/or are restricted by the extent of dye incorporation possible. Consequently, straightforward methods to covalently include dye molecules within step-growth commodity polymers that lack pendant functional groups, in the absence of coloured effluent generation, are urgently sought.
Polyesters may be employed within laundry detergent formulations as soil release polymers (SRPs), which aid the diffusion of water into the soil-fabric interface, decrease soil adsorption onto fabric surfaces, and reduce soil redeposition onto fabrics.13–15 SRPs must be amphiphilic to fulfil this role;16 the hydrophile ensures the dispersibility of the SRP in aqueous solution, whilst the lipophile facilitates the interaction of the SRP with the soil prior to soil removal from the garment.17 The inclusion of such materials within detergent formulations is crucial if the aim of low temperature laundering is to be achieved.18
As white fabric ages it adopts a yellow shade that remains following laundering.19–21 Attempts have been made to remedy this discolouration by the addition of trace quantities of blue dye, typically ultramarine blue.19 In modern times, such agents have adopted the synonymous terms optical brighteners and fluorescent whitening agents, with their primary purpose being the absorption of ultraviolet (UV) light and the fluorescence of blue light.22–24
Combining a blue dye that acts as an optical brightener with an SRP yields a material that holds great promise for application in advanced laundry applications as an active component that can both prevent fabric staining and discolouration, and impart brilliance to white fabrics. However, the permanent and extensive coloration of polymers that inherently lack repeat unit functionality is challenging. In addition, the conventional coupling of dye molecules to a polymer backbone results in inevitable non-coupling of dye molecules, leading to coloured wastewater and high lightfastness of the coloured material.
We report the permanent colouration of an amphiphilic polymer through innovative in situ chromophore creation. The simple addition of limited quantities of N-phenyldiethanolamine to the step-growth polymerisation yields a polymer that contains an aromatic amine capable of undergoing sequential diazotization and azo coupling reactions. Resultantly, the polymer only becomes coloured following chromophore formation upon azo bond formation, and the successful conjugation of a non-coloured molecule. Although we demonstrate the effectiveness of the polymers created within the context of laundry detergent active ingredients, this method of polymer colouration has wide-ranging potential for the effective colouration of a variety of commodity polyesters that are produced by step-growth polymerisation.
To achieve the aim of covalent step-growth polymer colouration, an amphiphilic polymer containing central ethylene terephthalate repeat units, terminated by hydrophilic poly(ethylene glycol) monomethyl ether (PEGMME) blocks (Fig. S1, ESI†), was modified to include an aniline group that may act as a handle for polymer modification by diazonium formation, and subsequent azo coupling reactions (P1) (Scheme 1a). With the target application of producing an SRP in mind, it was anticipated that the hydrophobicity provided by the central polyester units facilitates soil-polymer and/or fabric-polymer interactions, whilst the terminal, hydrophilic PEGMME units enable the polymer to disperse in aqueous solution. Chromophore formation only occurs upon diazenyl formation and so any discharge following azo coupling is uncoloured. The polymerisation to form P1 occurred over seven days at 210 °C. This reaction time far exceeds that of commercially produced PET, but the polymer produced contained terminal PEGMME blocks and presented a relatively low dispersity value of 1.4. It is envisaged that aniline-containing polyesters may be readily produced on an industrial scale following a procedure analogous to that of commercial PET synthesis.
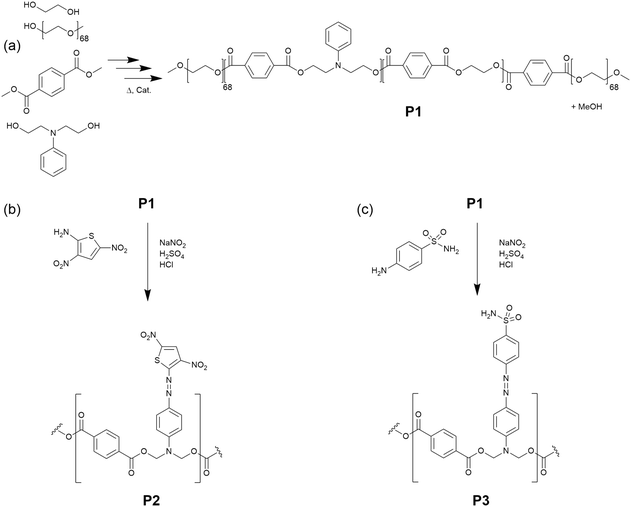
The successful synthesis of the uncoloured aniline-containing polymer backbone was confirmed by 1H NMR spectroscopy (Fig. S2a, ESI†). Peaks corresponding to the protons of PEGMME (h) are present between 3.24 and 3.89 ppm, and peaks corresponding to the ethylene glycol group in the terephthalate block (f) appear between 4.43 and 4.53 ppm. The protons corresponding to N-phenyldiethanolamine are represented by peaks between 6.63 and 7.17 ppm (b–d), 3.90 and 3.95 ppm (g) and 4.62 and 4.71 ppm (e) whilst peaks corresponding to the aromatic groups of the terephthalate group appear between 7.89 and 8.14 ppm (a). Theoretically, there are six times as many terephthalate groups to N-phenyldiethanolamine groups in the polymer formed, which was confirmed by comparing the integrals of the peaks corresponding to N-phenyldiethanolamine with the integrals of the aromatic protons corresponding to the terephthalate group. FTIR spectroscopy confirmed the presence of both ester and ether groups within P1 (Fig. 1a). In addition, aromatic peaks may be assigned within the fingerprint region at 842 cm−1 and 731 cm−1. Advanced polymer chromatography, a form of size exclusion chromatography, revealed that the polymer possessed average Mw and Mn values of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 7300 g mol−1, respectively, relative to poly(methyl methacrylate) standards (Fig. S3, ESI†).
000 g mol−1 and 7300 g mol−1, respectively, relative to poly(methyl methacrylate) standards (Fig. S3, ESI†).
To determine the effectiveness of P1 as an SRP, the polymer was tested for its capability to inhibit the transfer of indigo dye to a variety of common fabrics. An aqueous solution of P1 was prepared and added to a laboratory laundry wash simulator (laundrometer) together with an indigo dye bleed and strips of fabric (cellulose, cotton, wool, nylon, polyester and acrylic).
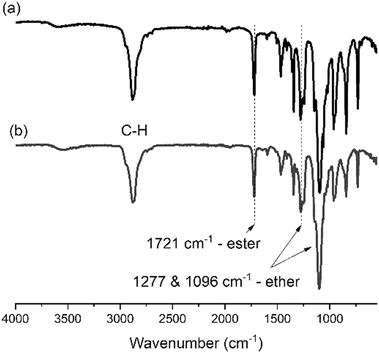
Domestic laundry washing was then simulated at 40 °C for 30 minutes in triplicate. A control simulated wash was also conducted in triplicate under the same conditions but without P1. The colour of the fabrics was measured using a spectrophotometer providing L*a*b* colour system values before and after washing. The colour difference (ΔE) was calculated as per eqn (1), whereby the lower the change in colour of the fabric after washing, the greater the extent of the polymer's capability to prevent dye transfer to the particular fabric type. The minimal addition of P1 to the simulated wash (only 0.1 mg mL−1 of P1 in 50 mL deionised water) vastly inhibited dye transfer to fabric during simulated washing, compared to when polymer was absent (Fig. 2). Dye transfer to nylon, polyester and woollen fabrics, was particularly restricted when P1 was included in the wash, compared to washes performed in the absence of the polymer. Such differences are clearly visible to the human eye.25
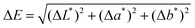 | (1) |
 | ||
| Fig. 2 The colour difference of various fabrics before and after simulated washing with an indigo dye bleed in the absence (control) and presence of P1. | ||
Textile shading dyes, which are included in detergents to provide low level colouration (typically blue or violet) to white fabric thus enhancing the human perception of whiteness, coupled to SRPs are a desirable prospect for use in laundry detergents. The use of a polymeric delivery vehicle may increase efficacy, homogeneity and the level of control over the dye interaction with the fabric surface. However, common dye-SRP approaches tend to yield high percentages of unattached dye either because the disperse dye used has not been able to react with the SRP or has undergone hydrolysis prior to conjugation with the SRP (Fig. S4, ESI†). Consequently, coloured wastewater is produced and the diffusion of dyes from fabric over time is a likely prospect.19 Permanent, covalent, colouration of P1 to yield a blue polymer (P2) was achieved (Scheme 1b) and the polymer characterised by 1H NMR spectroscopy (Fig. S2b, ESI†), FTIR spectroscopy (Fig. 1b) and UV-vis spectroscopy (Fig. S5, ESI†). The extent of blue dye release from the polymer into aqueous solution post-dyeing was monitored by UV-vis spectroscopy to determine if the system proposed restricts the unwanted release of dye molecules to solution. Using readily available materials, the recreation of commercially-available dye–polymer material that is suggested as a textile shading dye was realised for comparison.26 This comparative material follows the conventional approach of binding reactive dye molecules to an active acceptor site on the polymer, in this case the primary alcohol of the PEG block. Dialysis (membrane diffusion) was employed to resolve low molecular weight coloured components from both coloured polymers. The absorbance spectra of the outer dialysis media were collected and solved against a calibration curve (prepared using known concentrations of the pure unreacted/monomeric dye precursors) (Fig. S6, ESI†) to calculate the concentration of rogue dye present in the dye–polymer raw material. The conventional and novel approaches to coloured SRPs were found to contain unreacted dye contents of 18% and 2.3%, respectively (Fig. S7, ESI†), emphasising the effectiveness of the proposed method of polymer colouration.
It is important that dyeing moieties, such as optical brighteners, uniformly deposit on fabric to avoid discrepancies in the colour delivered (vs. intended) across mixed loads containing a variety of fabric types. The dye delivery of P2 and a commercially reported polymer–dye conjugate26 were compared by adding the optical brighteners independently to a laundrometer with cotton or polyester fabric and no dye bleed. The colour difference was calculated as per eqn (1) and the tests repeated to enable the consistency of dyeing to be quantified. Dye delivery to cotton and polyester was more consistent when P2 was employed compared to the conventional polymer–dye conjugate (Fig. S8a, ESI†). The same laundrometer tests were repeated with mixed washing loads containing cotton, polycotton and polyester fabrics, and it was found that P2 delivered significantly more consistent dyeing to the range of fabrics compared to the commercially reported polymer–dye conjugate (Fig. S8b, ESI†).
To demonstrate the versatility that the reported method of polymer colouration has, the conjugation of sulfanilamide to uncoloured P1 was done (Scheme 1c). The azo coupling reaction proceeded to yield a red solution immediately upon sulfanilamide addition to the polymer-containing solution (Fig. 3, top inset). The polymer obtained had a red colour (Fig. 3, bottom inset), that was confirmed by UV-vis spectroscopy in water (Fig. 3). The polymer was characterised by 1H NMR (Fig. S9, ESI†) and FTIR spectroscopy (Fig. S10, ESI†) to confirm successful polymer synthesis, and further demonstrate how the inclusion of limited amounts of N-phenyldiethanolamine within a step-growth polymerisation enables facile polymer colouration. Crucially, colouration of the polymer only occurred upon the creation of the azo group following the covalent coupling of sulfanilamide. The relative ease of P1 colouration ensures that scaled-up production within an industrial setting is anticipated to be straightforward.
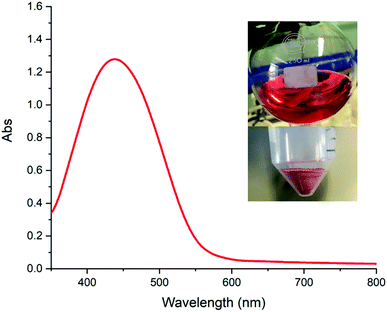 | ||
| Fig. 3 UV-Vis spectrum of P3. During the colouration, a red solution formed from which a light red polymer was precipitated. | ||
To conclude, an innovative method for the colouration of polymers produced by step-growth polymerisations involving dicarboxylic acid monomers, for instance polyesters, is demonstrated. The incorporation of N-phenyldiethanolamine within the step-growth polymerisation provides a molecular handle for prompt polymer colouration by a straightforward azo coupling reaction. This mode of permanent colouration ensures that a very limited amount of unbound dye molecules is expelled from the polymer post-synthesis. Initially, a water-soluble polymer that presented a blue hue was created and found to be effective as a vehicle for a blue shading dye, offering enhanced performance against a range of fabric types versus a commercially reported polymer–dye conjugate. Accordingly, the polymer has significant promise for incorporation within an advanced laundry care formulation to maintain the perception of whiteness of laundered items. The versatility of the mechanism of colour creation was demonstrated by the coupling of sulfanilamide to the uncoloured polymer, yielding a red polymeric product. It is envisaged that the simplistic method of polymer derivatisation and permanent colouration reported may be applied to a wide-range of polyesters created by step-growth polymerisation.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J.-H. Choi and W.-Y. Seo, Fibres Polym., 2006, 7, 270–275 CrossRef CAS.
- T. Salem, S. Uhlmann, M. Nitschke, A. Calvimontes, R.-D. Hund and F. Simon, Prog. Org. Coat., 2011, 72, 168–174 CrossRef CAS.
- A. D. Broadbent, Basic Principles of Textile Coloration, Society of Dyers and Colourists, Bradford, U.K., 2001 Search PubMed.
- T. Hussain, M. Tausif and M. Ashraf, J. Cleaner Prod., 2015, 108, 476–483 CrossRef CAS.
- S. M. Burkinshaw, J. Howroyd, N. Kumar and O. Kabambe, Dyes Pigm., 2011, 91, 340–349 CrossRef CAS.
- S. M. Burkinshaw, K. Liu and G. Salihu, Dyes Pigm., 2019, 171, 106367 CrossRef.
- A. Murcia-Salvador, J. A. Pellicer, M. I. Fortea, V. M. Gómez-López, M. I. Rodríguez-López, E. Núñez-Delicado and J. A. Gabaldón, Polymers, 2019, 11, 1003 CrossRef CAS PubMed.
- G. Moussavi and M. Mahmoudi, J. Hazard. Mater., 2009, 168, 806–812 CrossRef CAS PubMed.
- G. Mock, in Synthetic Fibre Dyeing, ed. C. Hawkyard, Society of Dyers and Colourists, Bradford, U.K., 2004, ch. 2, pp. 45–81 Search PubMed.
- T. Hayashi and P. D. Thornton, Dyes Pigm., 2015, 123, 304–316 CrossRef CAS.
- E. Zamani, H. Shaki, M. Rafizadeh, A. Khosravi and M. Pilehkouhi, Fibers Polym., 2017, 18, 1431–1437 CrossRef CAS.
- X. Hu, M. Li, Y. Xian, X. Liu, M. Liu, G. Li, P. Hu and C. Cheng, J. Appl. Polym. Sci., 2020, 137, 48862 CrossRef CAS.
- E. Kissa, in Detergency: Theory and Technology, ed. G. Cutler and E. Kissa, Marcel Dekker, Inc., New York, U.S.A., 1986, ch. 5, pp. 333–370 Search PubMed.
- B. S. Butola, in Polyesters and Polyamides, ed. B. L. Deopura, R. Alagirusamy, M. Joshi and B. Gupta, Woodhead Publishing Ltd., Cambridge, U.K., 2008, ch. 12, p. 325 Search PubMed.
- A. Valentini, S. Bakalis, K. Gkatzionis, G. Palazzo, N. Cioffi, C. D. Franco, E. Robles, A. Brooker and M. M. Britton, Ind. Eng. Chem. Res., 2019, 58, 14839–14847 CrossRef CAS.
- A. J. O'Lenick Jr., J. Surfactants Deterg., 1999, 2, 553–557 CrossRef.
- G. J. Stockburger, US Pat., US4427557, 1984 Search PubMed.
- L. Cotton, A. S. Hayward, N. J. Lant and R. S. Blackburn, Dyes Pigm., 2020, 177, 108120 CrossRef CAS.
- E. Smulders and E. Sung, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag, Weinheim, 2012, vol. 20, pp. 393–447 Search PubMed.
- I. Castro, E. Ekinci, X. Huang, H. A. Cheaito, Y.-H. Ahn, J. Olivero-Verbel and Q. P. Dou, J. Cell. Biochem., 2019, 120, 14065–14075 CrossRef CAS PubMed.
- H. Salas, C. Gutiérrez-Bouzán, V. López-Grimau and M. Vilaseca, Materials, 2019, 12, 785 CrossRef CAS PubMed.
- R. Chen, J. Qu, Q. Zhao and J. J. F. He, Fibres Polym., 2014, 15, 1915–1920 CrossRef CAS.
- Q. Zhao, J. Sun, B. Liu and J. J. C. He, Cellulose, 2014, 21, 2937–2950 CrossRef CAS.
- Z. Fang, F. Wu, L. Lin, Q. Qin, C. Au, Q. Tao, X. Li, D. Yu and B. Yi, J. Appl. Polym. Sci., 2019, 136, 47635 CrossRef.
- R. Baixauli, A. Salvador and S. M. Fiszman, Eur. Food Res. Technol., 2008, 226, 523–530 CrossRef CAS.
- S. N. Batchelor, J. M. Bird, W. Chen, S. Meng, Q. Tao and J. Wang, Int. Pat. App., WO2011098355, 2011.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental protocols, 1H NMR, and FTIR data. See DOI: 10.1039/d0cc00653j |
| This journal is © The Royal Society of Chemistry 2020 |