Novel small-molecule fluorophores for in vivo NIR-IIa and NIR-IIb imaging†
Qianqian
Li‡
ab,
Qihang
Ding‡
a,
Yang
Li
a,
Xiaodong
Zeng
 a,
Yishen
Liu
a,
Siyu
Lu
a,
Hui
Zhou
a,
Yishen
Liu
a,
Siyu
Lu
a,
Hui
Zhou
 a,
Xiaofei
Wang
c,
Junzhu
Wu
c,
Xianli
Meng
a,
Xiaofei
Wang
c,
Junzhu
Wu
c,
Xianli
Meng
 d,
Zixin
Deng
a and
Yuling
Xiao
d,
Zixin
Deng
a and
Yuling
Xiao
 *ab
*ab
aState Key Laboratory of Virology, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (MOE), Hubei Province Engineering and Technology Research Center for Fluorinated Pharmaceuticals, Wuhan University School of Pharmaceutical Sciences, Wuhan 430071, China. E-mail: xiaoyl@whu.edu.cn
bCollege of Science, Innovation Center for Traditional Tibetan Medicine Modernization and Quality Control, Medical College, Tibet University, Lasa, 850000, China
cHubei Provincial Key Laboratory of Developmentally Originated Disease, Center for Experimental Basic Medical Education, Wuhan 430071, China
dInnovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan 611137, China
First published on 5th February 2020
Abstract
Near-infrared fluorescence imaging in the 1000–1700 nm-wavelength window (NIR-II) has exhibited great potential for deep-tissue bioimaging due to its diminished auto-fluorescence, suppressed photo-scattering, deep penetration, and high spatial and temporal resolutions. Various kinds of inorganic nanomaterials have been extensively developed for NIR-IIa (1300–1400 nm) and NIR-IIb (1500–1700 nm) bioimaging. However, the development of small-molecule NIR-IIa and NIR-IIb fluorophores is still in its infancy. Herein, we designed and synthesized a novel NIR-II organic aggregation-induced emission (AIE) fluorophore (HQL2) with a fluorescence tail extending into the NIR-IIa and NIR-IIb region based on our previous reported skeleton Q4. The encapsulated NIR-II AIE nanoparticles (HQL2 dots) exhibited water solubility and biocompatibility, and high brightness for NIR-IIa and NIR-IIb vascular imaging in vivo, a first for NIR-II AIE dots.
Vascular diseases such as cardiocerebrovascular diseases and stroke are considered to be “top killers” and seriously affect the quality of life of people. Assessment of vascularity is crucial for achieving a precise diagnosis and treatment of such diseases. Up to now, various kinds of imaging techniques such as magnetic resonance imaging (MRI),1 Doppler ultrasonography,2 positron emission tomography (PET)3 and X-ray computer tomography (CT)4 have been widely used in clinical research. Recently, fluorescence imaging in the second near-infrared region (NIR-II, 1000–1700 nm) has been considered as a potential imaging technique, and has been widely studied for vascular imaging and tumor diagnosis due to its minimum light scattering, reduced auto-fluorescence and high penetration depth.5–12 Various inorganic and organic NIR-II materials, with maximum emission wavelengths below 1300 nm, have been developed for biomedical applications.13–30 Furthermore, molecular optical imaging in the NIR-IIa (1300–1400 nm) and NIR-IIb (1500–1700 nm) sub-windows displays nearly zero auto-fluorescence and a super resolution.31–35 So far, very few small-molecule NIR-IIa and NIR-IIb fluorophores for fluorescence imaging in the long-wavelength region have been reported.23,29–37 Of note, the organic dye FD-1080 was first employed for NIR-IIb vascular imaging with J-aggregates and showed a quantum yield of 0.0545%.37 Thus, developing a variety of small-molecule fluorophores having emission wavelenghts beyond 1300 and 1500 nm with high in vivo imaging quality and negligible background signals is indispensable. A potential way to develop such fluorophores is to make comprehensive use of the bright highly twisted NIR-II emitters with strong emission extending into the NIR-IIb region by following the aggregation-induced emission (AIE) strategy.38,39
Herein, the previously reported NIR-II molecule Q440 was modified by introducing dodecyl side chains into the different positions of the electron-rich thiophene spacer. Two highly twisted NIR-II dyes, HQL1 and HQL2, were obtained. Surprisingly, HQL2 showed high brightness and high quantum yield with AIE characteristics. Its maximum emission wavelength was ∼1050 nm, and the emission extended to 1600 nm. Then, HQL2 dots were prepared by encapsulating HQL2 with the amphiphilic molecule DSPE-PEG5K. The encapsulated NIR-II AIE HQL2 dots exhibited excellent photostability and biocompatibility, and were used for in vivo NIR-IIa and NIR-IIb vascular imaging. To the best of our knowledge, this is the first time that NIR-II AIE dots have been exploited in the NIR-IIa and NIR-IIb region for in vivo imaging.
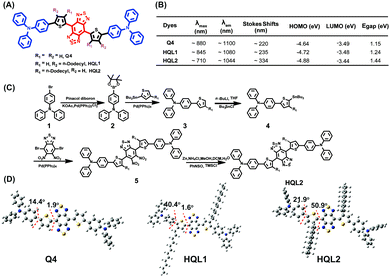
As illustrated in Fig. 1A, the fluorophores HQL1 and HQL2 employed a D–A–D structure with benzo-bis(1,2,5-thiadiazole (BBTD) as the acceptor, and a thiophene ring and Ph3N as the donor units. To minimize energy transfer and reduce intermolecular interactions between the fluorophores and the surrounding water, the hydrophobic alkyl group was integrated at the position R1 or R2. HQL1 and HQL2 were feasibly synthesized from Q4 according to the modified procedure (Fig. 1B and ESI†). Generally, the donor unit Ph3N conjugates to the thiophene unit first, and then couples to the acceptor BBTD to yield the desired products, mainly through Suzuki–Miyaura coupling, Stille coupling, Zn/NH4Cl reduction and N-thionylaniline-induced ring closure in 42–48.6% overall yields from the starting materials. All compounds were characterized using 1H-NMR, 13C-NMR and MALDI-TOF-MS or ESI-MS (Fig. S1–S14, ESI†), and showed excellent levels of solubility in various organic solvents.
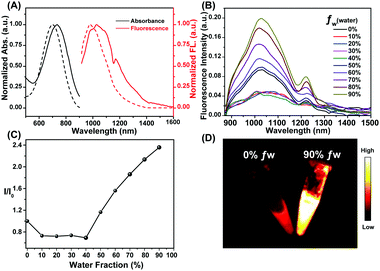
The highest occupied molecular orbitals (HOMOs), lowest unoccupied molecular orbitals (LUMOs) and energy gaps (Egaps) of these molecules calculated using Gaussian 09 software with 6-31G(d,p) basis sets revealed the importance of the dodecyl side chains at the different positions of thiophene. The substitution had strong effects on the molecular orbitals and molecular distortion. Positioning the dodecyl side chain close to the BBTD core increased the LUMO energy level and decreased that of the HOMO. All molecules showed optical bandgaps lower than 1.5 eV, which was regarded as one of the thresholds for NIR-II BBTD dyes. Upon photoexcitation of the S0 state, the molecular twist angle of HQL2 (50.9°) was much larger than those of HQL1 (1.6°) and Q4 (1.9°), revealing an order of distortion following the trend R1 = dodecyl, R2 = H > R1 = R2 = H > R1 = H, R2 = dodecyl between the thiophene donor and BBTD core (Fig. 1D and Fig. S15, ESI†). Various optical properties of HQL1 and HQL2 were also evaluated. HQL1 and HQL2 in dichloromethane (DCM) showed maximum emission wavelengths of ∼1080 nm and ∼1044 nm with emission QYs of 0.699% and 0.149%, respectively (Fig. S18 and S19, ESI†). Introduction of the molecular distortion at the R1 position caused a remarkable increase in fluorescence intensity with a 20–50 nm wavelength hypsochromic shift compared with Q4 (Fig. 1B and 2A). To determine the mechanism behind this phenomenon, the optical properties of HQL1 and HQL2 in various THF-water mixtures with different relative amounts of THF and water were investigated. The fluorescence intensity of HQL1 decreased as the relative amount of water was increased, indicative of obvious aggregation-caused quenching (ACQ) effects (Fig. S16, ESI†). In contrast, HQL2 showed a significant AIE effect (Fig. 2B and C). As shown in Fig. 2D, a strong fluorescence of HQL2 in a water/THF mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) was observed in comparison with that of HQL2 in pure THF. The molar absorption coefficients of HQL1 and HQL2 in DCM were determined to be 2.14 × 105 L mol−1 cm−1 and 1.98 × 104 L mol−1 cm−1, respectively. Based on these results, a tentative mechanism was proposed. According to this proposed mechanism, the inclusion of HQL2 resulted in a very strong fluorescence enhancement in the NIR-IIa and NIR-IIb windows, with its highly twisted backbone (i.e., having high dihedral angles) having reduced the intramolecular charge transfer (ICT)-induced fluorescence quenching effects, and increased the AIE-induced fluorescence enhancement effects using triphenylamine as a building block of AIEgens. These interesting results indicate that the novel light-up fluorophore HQL2 is suitable for in vivo NIR-IIa and NIR-IIb imaging.
1 v/v) was observed in comparison with that of HQL2 in pure THF. The molar absorption coefficients of HQL1 and HQL2 in DCM were determined to be 2.14 × 105 L mol−1 cm−1 and 1.98 × 104 L mol−1 cm−1, respectively. Based on these results, a tentative mechanism was proposed. According to this proposed mechanism, the inclusion of HQL2 resulted in a very strong fluorescence enhancement in the NIR-IIa and NIR-IIb windows, with its highly twisted backbone (i.e., having high dihedral angles) having reduced the intramolecular charge transfer (ICT)-induced fluorescence quenching effects, and increased the AIE-induced fluorescence enhancement effects using triphenylamine as a building block of AIEgens. These interesting results indicate that the novel light-up fluorophore HQL2 is suitable for in vivo NIR-IIa and NIR-IIb imaging.
HQL2 was then encapsulated in DSPE-PEG5K to form HQL2 dots according to the literature method.23 These dots have spherical shapes with an average particle size of ∼125.9 nm and hydrodynamic size of ∼155 nm, as determined from transmission electron microscopy (TEM) and dynamic light scattering (DLS) analyses, respectively (Fig. S21A and B, ESI†). The zeta potential of the HQL2 dots in PBS was determined to be −24.1 eV (Fig. S17, ESI†). The UV-Vis-NIR spectra of the HQL2 dots demonstrated peak absorption and emission wavelengths similar to those of HQL2, and tails into the 1600 nm wavelength region (Fig. S21C, ESI†). The molar absorption coefficient of HQL2 in water was measured to be ∼5.44 × 104 L mol−1 cm−1, 5-fold larger than that of HQL1 (ε: ∼1.06 × 104 L mol−1 cm−1) in water, demonstrating the greater brightness of HQL2 and its superiority for imaging. The fluorescence of the HQL2 dots (100 μg mL−1) could even be detected using a 1320 nm-wavelength long-pass (LP) filter and 1550 nm wavelength LP filter under an 808 nm wavelength excitation with extended exposure time (Fig. S21D, ESI†). The QY of the HQL2 dots was determined to be 1.19% in the NIR-II region (>1000 nm) in water with IR-26 (QY: 0.05%) as a reference, and 0.016% beyond 1300 nm, and 0.002% beyond 1550 nm (Fig. S18, ESI†).18,26 The HQL2 dots showed negligible decay in phosphate-buffered saline (PBS), fetal bovine serum (FBS) and water under 808 nm laser excitation for 60 min (180 mW cm−2, 10 ms exposure time) (Fig. S21E, ESI†), revealing the excellent photostability of these dots. The in vitro cell cytotoxicity of the HQL2 dots was studied in human hepatocyte L02 cell lines using the standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. As shown in Fig. S21F (ESI†), the cell viability of the L02 cells was much higher than ∼80% when incubated for 24 h even with a high concentration of HQL2 (80 μg mL−1). The blood circulation half-life was ∼226.5 min, determined by a quantification of the fluorescence intensity of HQL2 dots in the bloodstreams of female KM mice (n = 3) (Fig. S20, ESI†). These results showed a low cytotoxicity and good biocompatibility of the HQL2 dots, indicating the great potential of using these dots for in vivo NIR-IIa and NIR-IIb imaging.
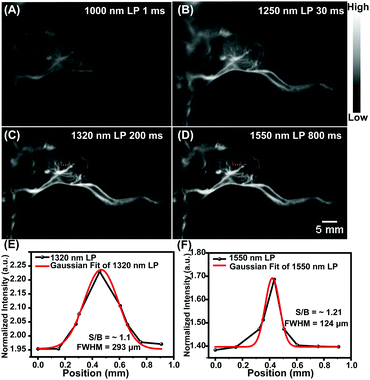
Real-time monitoring the dynamic change of blood vessels in the areas of lesions is of great significance for the diagnosis and treatment of cancer. In vivo NIR-IIa and NIR-IIb vascular imaging analyses were conducted by performing an intravenous injection of a sample of the HQL2 dots (200 μL, 5 mg mL−1) into C57 Balb/c (n = 3 per group) (Fig. 3A). The whole angiography was visualized using an InGaAs camera with different LP filters (1000, 1250, 1320 and 1550 nm) under 808 nm wavelength laser excitation (90 mW cm−2). The imaging of the HQL2 dots in the NIR-IIa window (1300–1400 nm, 1320 nm LP) with a 200 ms exposure time yielded superior resolution (Fig. 3A). An extended exposure time (800 ms) was required for high-resolution NIR-IIb imaging. The signal-to-background ratio (S/B) of the NIR-IIa imaging (S/B = 2.5, 1320 nm LP) was 2.5-fold higher than that of the NIR-II imaging (S/B = 1, 1000 nm LP and 1250 nm LP), and was also 1.4-fold higher than that of the NIR-IIb imaging (S/B = 1.8, 1550 nm LP) due to the low quantum yield beyond 1550 nm. The Gaussian-fitted full width at half maximum (FWHM) values of the vessels at the same position (in red dashed line) under different long-pass filters were calculated to be 2.13 mm (1000 nm LP), 1.05 mm (1250 nm LP), 0.345 mm (1320 nm LP) and 0.529 mm (1550 nm LP), respectively (Fig. 3B), demonstrating the great potential of using HQL2 dots for in vivo NIR-IIa and NIR-IIb imaging at extended exposure times.
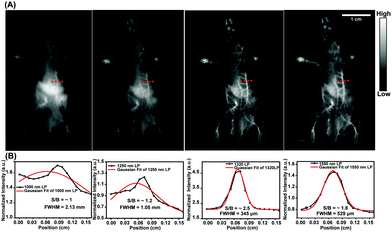 | ||
| Fig. 3 (A) The acquired fluorescence images of whole vessels in the abdomens of C57 Balb/c female mice using samples of the HQL2 dots under 808 nm-wavelength laser excitation (90 mW cm−2) with different long-pass filters (left to right: 1000, 1250, 1320, 1550 nm). (B) Cross-section intensity (black line) and Gaussian fit fluorescence intensity (red line) profiles with HQL2 dot imaging of the tiny vessels in Fig. 3A (red dashed line). | ||
Tumor angiogenesis can provide specific information for clinical research such as tumorigenesis and metastasis, and plays a crucial role in cancer diagnosis and treatment. For in vivo NIR-IIa and NIR-IIb tumor vessel imaging, samples of HQL2 dots (200 μL, 5 mg mL−1) were administered to U87MG-tumor-bearing female Balb/c nude mice (n = 3) through a tail vein injection. The tumor vascular imaging was performed immediately thereafter under an 808 nm laser excitation with various long-pass (LP) filters. The tumor vessels (red dashed line in Fig. 4C and D) were imaged clearly using the 1320 nm LP (NIR-IIa) and 1550 nm LP (NIR-IIb). The FWHM values of the tiny vessels (red dashed lines in Fig. 4C and D) were measured to be 0.293 mm (1320 nm LP) and 0.124 mm (1550 nm LP), respectively (Fig. 4E and F). In contrast, no desired tiny tumor vessels were detected using 1000 nm LP and 1250 nm LP owing to the relatively low resolution (Fig. 4A and B).
In summary, two novel NIR-II dyes, HQL1 and HQL2 based on our previous reported molecule Q4, were designed and synthesized. Inclusion of HQL2 with its large donor–acceptor distortion and strong NIR-II AIE yielded a remarkable increase in NIR-II fluorescence intensity. HQL2 dots encapsulated with DSPE-PEG5K showed strong fluorescence intensity beyond 1300 nm and 1550 nm with excellent photostability and biocompatibility, and were applied for in vivo NIR-IIa and NIR-IIb vascular and tumor vessel imaging under 808 nm-wavelength excitation. As far as we are concerned, the development of small-molecule fluorophores for bioimaging beyond 1300 nm and 1550 nm is still in its infancy. This novel NIR-II fluorophore HQL2 may nevertheless open up new approaches for the rational design of novel small molecules for NIR-IIa and NIR-IIb imaging with deep penetration and high resolution.
This work was partially supported by grants from the NSFC (81773674, 21473041, 81573383), the Project First-Class Disciplines Development Supported by Chengdu University of Traditional Chinese Medicine (CZYJC1903), the NSFHP (2017CFA0 24, 2016ACA126, 2017CFB711), the Tibet Autonomous Region Science and Technology Plan Project Key Project (XZ201901-GB-11), the Applied Basic Research Program of WMBST (2019020701011429), the Fundamental Research Funds for the Central Universities, and the Health Commission of Hubei Province Scientific Research Project (WJ2019M178, WJ2019M177).
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. Jacoby, Y. C. Böring, A. Beck, A. Zernecke, V. Aurich, C. Weber, J. Schrader and U. Flögel, J. Magn. Reson. Imaging, 2008, 28, 637–645 CrossRef PubMed.
- J. W. Wladimiroff, H. M. Tonge and P. A. Stewart, BJOG, 1986, 93, 471–475 CrossRef CAS PubMed.
- W. Chen and V. Dilsizian, J. Nucl. Med., 2015, 56, 503–504 CrossRef CAS PubMed.
- S. Kunjachan, J. Ehling, G. Storm, F. Kiessling and T. Lammers, Chem. Rev., 2015, 115, 10907–10937 CrossRef CAS PubMed.
- G. Hong, J. C. Lee, J. T. Robinson, U. Raaz, L. Xie, N. F. Huang, J. P. Cooke and H. Dai, Nat. Med., 2012, 18, 1841–1846 CrossRef CAS PubMed.
- C. Qu, Y. Xiao, H. Zhou, B. Ding, A. Li, J. Lin, X. Zeng, H. Chen, K. Qian, X. Zhang, W. Fang, J. Wu, Z. Deng, Z. Cheng and X. Hong, Adv. Opt. Mater., 2019, 7, 1900229 CrossRef.
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 0010 CrossRef CAS.
- G. Hong, J. T. Robinson, Y. Zhang, S. Diao, A. L. Antaris, Q. Wang and H. Dai, Angew. Chem., Int. Ed., 2012, 51, 9818 CrossRef CAS PubMed.
- C. Li, Y. Zhang, M. Wang, Y. Zhang, G. Chen, L. Li, D. Wu and Q. Wang, Biomaterials, 2014, 35, 393–400 CrossRef CAS PubMed.
- Y. Du, B. Xu, T. Fu, M. Cai, F. Li, Y. Zhang and Q. Wang, J. Am. Chem. Soc., 2010, 132, 1470–1471 CrossRef CAS PubMed.
- Y. Tang, F. Pei, X. Lu, Q. Fan and W. Huang, Adv. Opt. Mater., 2019, 7, 1900917 CrossRef CAS.
- Q. Wang, Y. Dai, J. Xu, J. Cai, X. Niu, L. Zhang, R. Chen, Q. Shen, W. Huang and Q. Fan, Adv. Funct. Mater., 2019, 29, 1901480 CrossRef.
- J. T. Robinson, G. Hong, Y. Liang, B. Zhang, O. K. Yaghi and H. Dai, J. Am. Chem. Soc., 2012, 25, 10664–10669 CrossRef PubMed.
- R. Wang, X. Li, L. Zhou and F. Zhang, Angew. Chem., Int. Ed., 2014, 53, 12086–12090 CrossRef CAS PubMed.
- J.-Y. Zhao, G. Chen, Y.-P. Gu, R. Cui, Z.-L. Zhang, Z.-L. Yu, B. Tang, Y.-F. Zhao and D.-W. Pang, J. Am. Chem. Soc., 2016, 138, 1893–1903 CrossRef CAS PubMed.
- S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC.
- Z. Liu, F. Ren, H. Zhang, Q. Yuan, Z. Jiang, H. Liu, Q. Sun and Z. Li, Biomaterials, 2019, 219, 119364 CrossRef CAS.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS.
- J. Yang, Q. Xie, H. Zhou, L. Chang, W. Wei, Y. Wang, H. Li, Z. Deng, Y. Xiao, J. Wu, P. Xu and X. Hong, J. Proteome Res., 2018, 17, 2428–2439 CrossRef CAS PubMed.
- H. Zhou, H. Yang, L. Tang, Y. Wang, Y. Li, N. Liu, X. Zeng, Y. Yan, J. Wu, S. Chen, L. Xiao, Y. Yu, Z. Deng, H. Deng, X. Hong and Y. Xiao, J. Mater. Chem. C, 2019, 7, 9448–9454 RSC.
- X. Zeng, Y. Xiao, J. Lin, S. Li, H. Zhou, J. Nong, G. Xu, H. Wang, F. Xu, J. Wu, Z. Deng and X. Hong, Adv. Healthcare Mater., 2018, 7, 1800589 CrossRef.
- R. Tian, H. Ma, Q. Yang, H. Wan, S. Zhu, S. Chandra, H. Sun, D. O. Kiesewetter, G. Niu, Y. Liang and X. Chen, Chem. Sci., 2019, 10, 326–332 RSC.
- J. Lin, X. Zeng, Y. Xiao, L. Tang, J. Nong, Y. Liu, H. Zhou, B. Ding, F. Xu, H. Tong, Z. Deng and X. Hong, Chem. Sci., 2019, 10, 1219–1226 RSC.
- X. Zeng, L. Xue, D. Chen, S. Li, J. Nong, B. Wang, L. Tang, Q. Li, Y. Li, Z. Deng, X. Hong, M. Wu and Y. Xiao, Chem. Commun., 2019, 55, 14287–14290 RSC.
- Z. Sheng, B. Guo, D. Hu, S. Xu, W. Wu, W. H. Liew, K. Yao, J. Jiang, C. Liu, H. Zheng and B. Liu, Adv. Mater., 2018, 30, 1800766 CrossRef PubMed.
- F. Ren, L. Ding, H. Liu, Q. Huang, H. Zhang, L. Zhang, J. Zeng, Q. Sun, Z. Li and M. Gao, Biomaterials, 2018, 175, 30–43 CrossRef CAS PubMed.
- Y. Xu, F. Ren, H. Liu, H. Zhang, Y. Han, Z. Liu, W. Wang, Q. Sun, C. Zhao and Z. Li, ACS Appl. Mater. Interfaces, 2019, 11, 21399–21407 CrossRef CAS.
- B. Ding, Y. Xiao, H. Zhou, X. Zhang, C. Qu, F. Xu, Z. Deng, Z. Cheng and X. Hong, J. Med. Chem., 2019, 62, 2049–2059 CrossRef CAS.
- X. Zeng, Z. Chen, L. Tang, H. Yang, N. Liu, H. Zhou, Y. Li, J. Wu, Z. Deng, Y. Yu, H. Deng, X. Hong and Y. Xiao, Chem. Commun., 2019, 55, 2541–2544 RSC.
- S. Wang, Y. Fan, D. Li, C. Sun, Z. Lei, L. Lu, T. Wang and F. Zhang, Nat. Commun., 2019, 10, 1058 CrossRef PubMed.
- Z. Zhang, X. Fang, Z. Liu, H. Liu, D. Chen, S. He, J. Zheng, B. Yang, W. Qin, X. Zhang and C. Wu, Angew. Chem., Int. Ed., 2019 DOI:10.1002/anie.201914397.
- Z. Xue, S. Zeng and J. Hao, Biomaterials, 2018, 171, 153–163 CrossRef CAS PubMed.
- S. Wang, L. Liu, Y. Fan, A. M. El-Toni, M. S. Alhoshan, D. Li and F. Zhang, Nano Lett., 2019, 19, 2418–2427 CrossRef CAS PubMed.
- Z. Deng, X. Li, Z. Xue, M. Jiang, Y. Li, S. Zeng and H. Liu, Nanoscale, 2018, 10, 9393–9400 RSC.
- Y. Li, S. Zeng and J. Hao, ACS Nano, 2019, 13, 248–259 CrossRef CAS PubMed.
- Y. Zhong, Z. Ma, S. Zhu, J. Yue, M. Zhang, A. L. Antaris, J. Yuan, R. Cui, H. Wan, Y. Zhou, W. Wang, N. F. Huang, J. Luo, Z. Hu and H. Dai, Nat. Commun., 2017, 8, 737 CrossRef PubMed.
- C. Sun, B. Li, M. Zhao, S. Wang, Z. Lei, L. Lu, H. Zhang, L. Feng, C. Dou, D. Yin, H. Xu, Y. Cheng and F. Zhang, J. Am. Chem. Soc., 2019, 141, 19221–19225 CrossRef CAS PubMed.
- S. Liu, X. Zhou, H. Zhang, H. Ou, J. W. Y. Lam, Y. Liu, L. Shi, D. Ding and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 5359–5368 CrossRef CAS PubMed.
- Q. Yang, Z. Hu, S. Zhu, R. Ma, H. Ma, Z. Ma, H. Wan, T. Zhu, Z. Jiang, W. Liu, L. Jiao, H. Sun, Y. Liang and H. Dai, J. Am. Chem. Soc., 2018, 140, 1715–1724 CrossRef CAS.
- Y. Sun, C. Qu, H. Chen, M. He, C. Tang, K. Shou, S. Hong, M. Yang, Y. Jiang, B. Ding, Y. Xiao, L. Xing, X. Hong and Z. Cheng, Chem. Sci., 2016, 9, 6203–6207 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc09865h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |