Photoanodes for water oxidation with visible light based on a pentacyclic quinoid organic dye enabling proton-coupled electron transfer†
Abstract
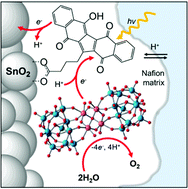
A pentacyclic quinoid dye, KuQ(O)3OH, combining (i) extended visible absorption up to 600 nm, (ii) excited state reduction potential >2 V vs. NHE, and (iii) a photoinduced proton-coupled electron transfer mechanism, has been used for the fabrication of dye-sensitized SnO2 photoanodes integrating a ruthenium polyoxometalate water oxidation catalyst. The resulting photoelectrode SnO2|KuQ(O)3OH|Ru4POM displays a light harvesting efficiency up to 90% in the range 500–600 nm, an onset potential as low as 0.2 V vs. NHE at pH 5.8, photoinduced oxygen evolution with a faradaic efficiency of 70 ± 15% and an absorbed-photon-to-current efficiency up to 0.12 ± 0.01%.


 Please wait while we load your content...
Please wait while we load your content...