A fluorescent naphthalimide NADH mimic for continuous and reversible sensing of cellular redox state†
Abstract
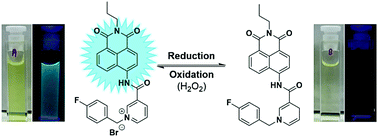
A fluorescent, naphthalimide-based, NADH mimic has been synthesised as a reversible, biocompatible, “on–off” probe for the detection of changes in intracellular redox environment (both oxidation and reduction). Interconversion was confirmed by means of electrochemistry and also 1H NMR, UV-vis and fluorescence spectroscopy. The reversibility was also successfully detected in A549 cells under simulated redox stress.
- This article is part of the themed collection: The Mechanics of Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...