An ultrafast responsive NO2 gas sensor based on a hydrogen-bonded organic framework material†
Abstract
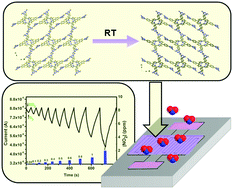
We report the development of a new type of organic semiconductor gas sensor based on a porphyrin-based hydrogen-bonded organic framework (HOF). Owing to the orderly porous structures, the decoration with rich amino sites and the n-type semiconductor nature, this HOF-based sensor exhibits selective NO2 sensing performance with ultra-fast response/recovery rates (17.6 s/15.4 s over 100 ppb) and a limit of detection lower than 40 ppb, together with high sensitivity, good reproducibility, and long-term stability at room temperature. This study demonstrates that HOF-based materials have potential application prospects in gas sensing, thereby offering a new way of thinking for the design and development of sensors.


 Please wait while we load your content...
Please wait while we load your content...