Why do silanes reduce electron-rich phosphine oxides faster than electron-poor phosphine oxides?†
Abstract
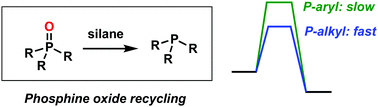
Organophosphine-mediated reactions that generate P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-bonded byproducts can be transformed into catalytic processes by reducing the R3P
O-bonded byproducts can be transformed into catalytic processes by reducing the R3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O byproduct back to PR3in situ with a silane. DFT calculations explain why the most readily reduced phosphine oxides are those incorporating electron-rich (e.g. alkyl) substituents rather than electron-deficient (e.g. aryl) substituents.
O byproduct back to PR3in situ with a silane. DFT calculations explain why the most readily reduced phosphine oxides are those incorporating electron-rich (e.g. alkyl) substituents rather than electron-deficient (e.g. aryl) substituents.


 Please wait while we load your content...
Please wait while we load your content...