Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Janus bis(NHCs) tuned by heteroatom-bridge oxidation states†
Nabila Rauf
Naz
a,
Gregor
Schnakenburg
 a,
Antal
Mikeházi
a,
Antal
Mikeházi
 b,
Zsolt
Kelemen
b,
Zsolt
Kelemen
 b,
László
Nyulászi
b,
László
Nyulászi
 *b,
René T.
Boeré
*b,
René T.
Boeré
 c and
Rainer
Streubel
c and
Rainer
Streubel
 *a
*a
aInstitut für Anorganische Chemie der Rheinischen Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany. E-mail: r.streubel@uni-bonn.de; Web: http://anorganik.chemie.uni-bonn.de/akstreubel
bDepartment of Inorganic and Analytical Chemistry and MTA-BME Computation Driven Chemistry Research Group, Budapest University of Technology and Economics, Szt Gellert ter 4, 1111 Budapest, Hungary. E-mail: nyulaszi@mail.bme.hu
cDepartment of Chemistry and Biochemistry, University of Lethbridge, 4401 University Drive West, Lethbridge, AB T1K3M4, Canada. E-mail: boere@uleth.ca
First published on 13th January 2020
Abstract
Synthesis of the first tricyclic bis(carbenes) with facially opposed imidazole-2-ylidenes and two linking phosphorus centres in different oxidation states is presented using a modular, high-yield synthetic route. The formation of homo bimetallic coinage metal complexes provides a glimpse on their potential use.
N-Heterocyclic carbenes (NHCs) I were proposed as transient species more than a half century ago,1 but it was Arduengo2a who achieved the breakthrough of synthesizing the first stable cyclic carbene, following closely on the first isolable carbene stabilized by PIII.2b This achievement initiated an impressive development in imidazole-2-ylidene chemistry (Fig. 1), and beyond.3 More recently, NHC chemistry became sophisticated enough to build ligands possessing structural diversities and catalytic functionalities,4 applicable in coordination chemistry,5 homogeneous catalysis,6 and organocatalysis.7 Modification of electronic properties of NHCs, became a primary issue and various concepts were followed, i.e., N-substituent design or annellation with the imidazole ring,8 but also to exert electronic influence9via NHC backbone substituents.10
 | ||
| Fig. 1 Mono NHCs I and II, Bielawski bis(NHCs) III and E-bridged bis(NHCs) IV–VI (E = main group element). | ||
In case of the latter, the initial focus was on mono-NHCs bearing substituents derived from heteroatoms such as Cl,11 O,12 N,13 Si,14 B,15 and P.16 In contrast, knowledge about [a,d]benzannulated “ditopic” Janus bis(NHCs) III, first reported by Bielawski et al.17 remains scarce. Coordination properties were investigated by Peris and used to build novel organometallic architectures.18 Hahn and co-workers created molecular squares and quadrilaterals in supramolecular assemblies.19 Mono-NHCs I and flexible bis(NHCs) IV having phosphorus as heteroatom bound to the backbone and possessing different oxidation states were studied,16,20 but rigid, heteroatom-linked bis(NHCs) such as V and VI (Fig. 1) are almost unknown.20c This is surprising as the incorporation of hetero-atoms would not just enable unprecedented chemical diversity but also a functional entity that could be redox active and/or possess further, different donor centres. The recent report on tricyclic 1,4-dihydro-1,4-diphosphinines21 bearing dithione functionalities constituted an interesting conceptional starting point for the synthesis of P-functional bis(NHCs).
Herein, synthesis of the first tricyclic PV/V- and PIII/III-bridged bis(imidazole-2-ylidenes) and their use to form dinuclear coinage metal(I) complexes is reported. Structural and electronic properties of the free ligands and their bimetallic complexes are discussed using combined DFT and CV results.
As our initial studies of the oxidative desulfurization of tricyclic 1,4-dihydro-1,4-diphosphinine dithiones failed, we considered synthesizing and employing the corresponding diselones. The tricyclic PIII/III-bridged diselone 1 was obtained as cis/trans mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7) in good yields after work-up using previously published protocols.21,22 In this case we could also separate the cis and trans isomers using low-temperature column chromatography, thus being able to fully characterize 1cis and, hence, assigning the 31P resonance signal at 0.91 ppm to the cis, and at 3.75 ppm to the trans isomer. 1cis was also structurally confirmed (see ESI†). Starting from 1cis/trans and using 10 eq. of H2O2 in dichloromethane, the isomeric PV/V-bridged bis(imidazolium) salts (1
0.7) in good yields after work-up using previously published protocols.21,22 In this case we could also separate the cis and trans isomers using low-temperature column chromatography, thus being able to fully characterize 1cis and, hence, assigning the 31P resonance signal at 0.91 ppm to the cis, and at 3.75 ppm to the trans isomer. 1cis was also structurally confirmed (see ESI†). Starting from 1cis/trans and using 10 eq. of H2O2 in dichloromethane, the isomeric PV/V-bridged bis(imidazolium) salts (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9) were formed via oxidative deselenization,23 and immediate treatment with BaCl2·2H2O led to the chloride salts 2cis/trans (Scheme 1). The oxidation of the PIII/III to PV/V centres was revealed by 31P resonances of δ = −6.2 (cis) and −5.6 (trans). Formation of the PV/V bis(imidazolium) derivative 2cis/trans was confirmed by various analytical methods and an XRD structure (see ESI†).
0.9) were formed via oxidative deselenization,23 and immediate treatment with BaCl2·2H2O led to the chloride salts 2cis/trans (Scheme 1). The oxidation of the PIII/III to PV/V centres was revealed by 31P resonances of δ = −6.2 (cis) and −5.6 (trans). Formation of the PV/V bis(imidazolium) derivative 2cis/trans was confirmed by various analytical methods and an XRD structure (see ESI†).
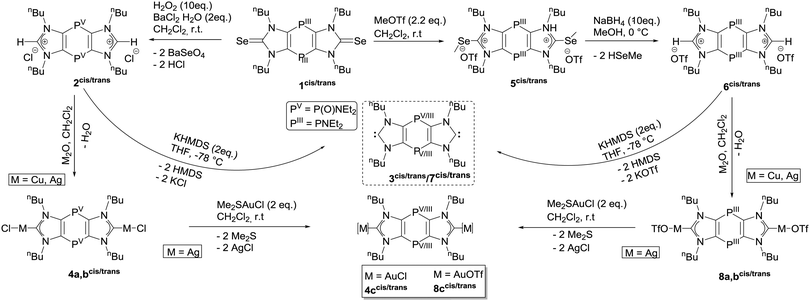 | ||
| Scheme 1 Synthesis of PV/V/PIII/III bridged bis(imidazolium) salt 2cis/trans/6cis/trans, PV/V/PIII/III bis(NHC) 3cis/trans/7cis/trans and their coinage metal(I) complexes 4a–ccis/trans/8a–ccis/trans. | ||
Tricyclic PV/V-bridged bis(imidazolium) salts 2cis/trans were converted into PV/V-bis(NHCs) 3cis/trans (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) in overall 88% yield by deprotonation using two eq. of KHMDS in THF (Scheme 1). The 31P{H} NMR spectrum of 3cis/trans revealed resonances at −2.4 (cis) and −1.2 (trans) ppm, only slightly downfield shifted compared to 2cis/trans. The absence of C2-proton resonances in the 1H NMR spectrum and the typical downfield shift of the C2-carbon resonances at 225.0 ppm17 in the 13C{1H} NMR spectrum of 3cis/trans provided firm NMR spectroscopic evidence for the biscarbenes (for further details see ESI†).
0.2) in overall 88% yield by deprotonation using two eq. of KHMDS in THF (Scheme 1). The 31P{H} NMR spectrum of 3cis/trans revealed resonances at −2.4 (cis) and −1.2 (trans) ppm, only slightly downfield shifted compared to 2cis/trans. The absence of C2-proton resonances in the 1H NMR spectrum and the typical downfield shift of the C2-carbon resonances at 225.0 ppm17 in the 13C{1H} NMR spectrum of 3cis/trans provided firm NMR spectroscopic evidence for the biscarbenes (for further details see ESI†).
The molecular structure of 3trans (Fig. 2) possesses an N1–C3–N2 bond angle of 102.4(11)°, slightly more acute than in (imidazole-2-ylidenes) I (R = Ad or tBu, 104.4° or 104.8°).24
M06-2X/6-31+G* DFT calculations on 3′cis/trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 indicate a 0.5 kcal mol−1 preference for the trans isomer. For both isomers (3′cis/trans), the π-type LUMO (Fig. 4) is delocalized over the central ring and the HOMO (ε = −6.17 eV) for the trans isomer and ε = −6.19 eV for the cis isomer are antibonding combinations of the two weakly coupled (Fig. S55 of the ESI†) in-plane carbene lone pairs (Fig. 4). In accordance with the acute bond angle, they have strong s-character (51.7% at B3LYP/6-31+G**//M06-2X/6-31+G*, comparable to 53.0% in PH3, see Fig. 4). To assess the stability of the tricyclic carbenes, we investigated the isodesmic reaction: R′R′′C: + CH4 → R′R′′CH4 + CH2 for 3′cis/trans.26 For (N-methyl)imidazole-2-ylidene, 111.5 kcal mol−1, for 3′cis 113.3, and for 3′trans 111.7 kcal mol−1 stabilization was obtained.
25 indicate a 0.5 kcal mol−1 preference for the trans isomer. For both isomers (3′cis/trans), the π-type LUMO (Fig. 4) is delocalized over the central ring and the HOMO (ε = −6.17 eV) for the trans isomer and ε = −6.19 eV for the cis isomer are antibonding combinations of the two weakly coupled (Fig. S55 of the ESI†) in-plane carbene lone pairs (Fig. 4). In accordance with the acute bond angle, they have strong s-character (51.7% at B3LYP/6-31+G**//M06-2X/6-31+G*, comparable to 53.0% in PH3, see Fig. 4). To assess the stability of the tricyclic carbenes, we investigated the isodesmic reaction: R′R′′C: + CH4 → R′R′′CH4 + CH2 for 3′cis/trans.26 For (N-methyl)imidazole-2-ylidene, 111.5 kcal mol−1, for 3′cis 113.3, and for 3′trans 111.7 kcal mol−1 stabilization was obtained.
The NICS(0) values of the imidazole units are −10.5 for the cis and −10.9 for the trans isomer, indicating slightly reduced aromaticity compared to the parent imidazole-2-ylidene (NICS(0) = −11.3) middle ring is about nonaromatic as indicated by the small positive NICS(0) values (0.5 for 3′cis and 0.1 for 3′trans). Also the 89.9(cis) / 94.1(trans) measured 77Se chemical shift of derivative 3cis/trans = Se, is somewhat more negative than for 1,3-diisopropyl-imidazole-2-selone (−3 ppm), and comparable to 1,3-dipp-imidazole-2-selone (87.0 ppm).27
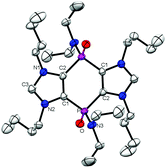
The PV/V-bridged bis(imidazolium) salts 2cis/trans reacted in dichloromethane with one eq. of M2O (M = Cu, Ag) led to PV/V-bridged bis(NHC complexes) 4a–bcis/trans while 4ccis/trans was synthesized by metal exchange reaction with complex 4bcis/trans. All complexes 4a–ccis/trans were obtained as white powders and were fully characterized (see ESI† and Table 1). The molecular structure of 4ctrans from XRD (Fig. 3) shows an almost perfect colinear arrangement of the two C2–Au1 bonds of different molecules. The C2–Au1–Cl1 axis seem to deviate only slightly from linearity (cf.ref. 11) and the trans-parallel orientation of two molecules of complex 4ctrans molecules indicate intermolecular aurophilic interactions in the solid state. Similar to the parent system, the trans isomer of the Cu complex 4a′ is calculated (at M06-2X/6-31+G*) to be more stable by 0.4 kcal mol−1, which holds true for all the metal complexes (Cu, Ag and Au).
| δ(31P)/ppm (CD2Cl2) | δ(13C)/ppm (CD2Cl2)c | Ratiod | |
|---|---|---|---|
| a In case of 3cis/trans, C6D6. b 7cis/trans, THF-d8. c C2 carbon. d (cis/trans) ratios. | |||
| 2cis/trans | −6.2 (cis), −5.9 (trans) | 146.5 (d, 3JP,C = 12.8 Hz) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9 |
| 6cis/trans | 5.4 (cis), 5.8 (trans) | 141.0 (s) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
3cis/trans
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
|
−2.4 (cis), −1.2 (trans) | 225.0 (t, 3JP,C = 2.7 Hz) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 0.7 |
7cis/trans
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
|
6.7(cis), 7.0 (trans) | 220.2 (t, 3JP,C = 2.4 Hz) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
 | ||
| Fig. 3 Molecular structure of compound 4ctrans (ellipsoids at the 50% probability level) and hydrogen atoms are omitted for clarity (see ESI†). | ||
It was obvious that the same protocol could not be used to access free PIII/III-bridged bis(NHCs). Therefore, an initial two-fold Se-methylation of 1cis/trans was considered.20a The salt 5cis/trans was easily obtained (84%, ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.33) if 2 eq. of trifluoromethyl sulfonate (MeOTf) were used; for further details see ESI.† Subsequent use of 5cis/trans and 5 eq. of Na[BH4] in methanol resulted in the formation of PIII/III-bridged bis(imidazolium) salts 6cis/trans which could be isolated as an orange liquid (61%, 1
0.33) if 2 eq. of trifluoromethyl sulfonate (MeOTf) were used; for further details see ESI.† Subsequent use of 5cis/trans and 5 eq. of Na[BH4] in methanol resulted in the formation of PIII/III-bridged bis(imidazolium) salts 6cis/trans which could be isolated as an orange liquid (61%, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3) and were fully characterized; for selected NMR data, see Table 1.
0.3) and were fully characterized; for selected NMR data, see Table 1.
The facile access to 6cis/trans prompted us to target the PIII/III-bridged bis(NHC) 7cis/trans using 2 eq. of KHMDS in THF. The 31P{1H} NMR spectrum of the reaction mixture shows a slight downfield shift for the resonances of the new product 7cis/trans.
As for 3′cis/trans the energy difference between the two isomers of 7′cis/trans is small (0.8 kcal mol−1), however, in case of 7′ the cis isomer is the more stable one. The inversion barrier of the phosphorus is high (44.2 kcal mol−1), thus isomerization cannot be expected at room temperature. The stabilization energy of 7′ (111.1 kcal mol−1 for cis and 109.2 kcal mol−1 for trans) is also close to those of the parent imidazole-2-ylidene and 3′.26 The aromaticity of the imidazole ring decreased somewhat according to the NICS(0) values (−9.2 for cis and −9.5 for trans) compared to 3′cis/trans (see NICS(1) values in the ESI†). On the other hand the aromatic character of the middle ring increased slightly, which was indicated by the negative (although small) NICS(0) values (−0.5 for 7′cis and −0.9 for 7′trans). Oxidation of PIII centers was shown to increase antiaromaticity in phospholes,28 due to the increased involvement of σ* orbitals, which are significantly lower in energy for the PV system.29 Though the shape and the localization of HOMO and the LUMO of 7′cis/trans are similar to 3′cis/trans, their energy levels are somewhat stabilized (see Fig. 4). Interestingly, the stabilization of the LUMO of 3′cis/trans with respect to 7′cis/trans has little effect on the electron acceptor property of the NHC since the carbene atoms are not involved in the LUMO.
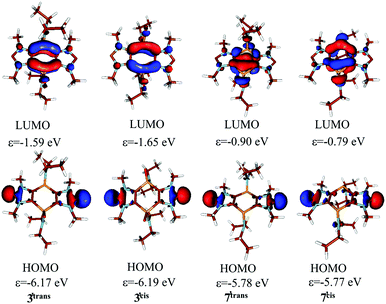 | ||
| Fig. 4 Kohn–Sham frontier orbitals of 3′ and 7′ and their energies at B3LYP/6-31+G*//M06-2X/6-31+G* level of theory. | ||
Indeed, the 35.9 (cis)/37.9 (trans) 77Se chemical shift of 7cis/trans is closer to that of 1,3-diisopropyl-imidazole-2-selone (−3 ppm), than in case of 3cis/trans (89.9/94.1 ppm – see above) showing that the P(V) substitution increases somewhat the electron acceptor ability of the carbene; however, still within the known range of imidazole-2-ylidenes.27
To examine the different coordination sites of bis(NHC) 7cis/trans, reactions with coinage metals were undertaken. Thus, salts 6cis/trans were treated with M2O (M = Cu, Ag) which resulted in the clean formation of PIII/III-bridged bis(NHC) complexes 8a–bcis/trans (Table S2, ESI†). 8c was obtained by metal exchange reaction using dimethyl sulfide gold(I) chloride. From the 31P{1H} NMR spectra of the reaction solutions it became immediately apparent that the strategy was successful and no binding had occurred to the PIII/III centers as their resonance signals just slightly changed compared to 6cis/trans. The formation of the M–C2 bond is in good agreement with the calculated Kohn–Sham orbitals as the lone pairs of the phosphorus do not have any contribution to the HOMO of 7cis/trans (Fig. 4). The highfield shift of the C2 carbon resonances in the 13C{1H} NMR spectra indicate clearly the C2 binding of the metal(I) centers and, hence, formation of complexes 8a–ccis/trans; the latter were also confirmed via pos. and neg. ESI-MS experiments (see also ESI†).
The donor properties of dicarbenes 3cis/trans and 7cis/trans were investigated by voltammetry. All undergo facile oxidation processes that are strongly solvent-dependent as noted previously in other cases of mono-NHCs.30 Cyclic voltammograms (CVs) measured on gold ceramic screen printed electrodes display for 3cis/transEap1 = −0.16 V vs. Fc+/0 (Fc = ferrocene) in CH2Cl2 and −0.45 V in THF and for 7cis/transEap1 = −0.30; Ecp1 = −0.42 V; ΔE = 120 mV; Em = −0.36 V. The return peak is not observed when scanning further positive and all species display multiple oxidations up to the solvent limits. For 7cis/transEap1 = −0.61 V in THF. There is no evidence of differentiation of the CV peaks between the cis and trans isomers. Cobaltocenium (Cc) in the form of [Cc][PF6] was a suitable internal standard in CH2Cl2. Both the low first and multiple further oxidations are supported by the DFT calculations (high-energy HOMOs with carbene-C δ character and several other occupied orbitals close to the HOMOs). Moreover, the more facile oxidation of 7cis/trans is in good agreement with the ∼0.4 eV higher computed energy of the HOMOs of 7′cis/transversus3′cis/trans.
The first examples of Janus heteroatom-bridged bis-(NHCs) with variable oxidation states of the bridging atoms are reported. The (PIII)2 bridge induces a more basic carbene center and moreover opens the possibility of exploiting a tetratopic ligand; the latter may also hold for the (PV)2 bridged bis(NHC). This multigram synthetic approach may allow for the introduction of other p-block bridging elements to further expand the potential for Janus-type bis(NHC) ligands in coordination chemistry. But it may also stimulate the rapidly developing field of NHC main group element adduct chemistry.31
We are grateful to the University of Bonn for financial support. L. N. and R. T. B. are grateful for the Alexander von Humboldt Stiftung for the re-invitation, L. N. for the NKFIH OTKA NN 113772 and R. T. B. to NSERC-Canada.
Conflicts of interest
There are no conflicts of interest.References
- (a) R. Breslow, J. Am. Chem. Soc., 1957, 79, 1762–1763 CrossRef CAS; (b) H. W. Wanzlick and E. Schikora, Angew. Chem., 1960, 72, 494 CrossRef CAS; (c) P. Haake and W. B. Miller, J. Am. Chem. Soc., 1963, 85, 4044–4045 CrossRef CAS.
- (a) A. J. Arduengo III, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361–363 CrossRef; (b) A. Igau, H. Grützmacher, A. Baceiredo and G. Bertrand, J. Am. Chem. Soc., 1988, 110, 6463–6466 CrossRef CAS.
- (a) V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt and S. Inoue, Coord. Chem. Rev., 2018, 118, 9678–9842 CrossRef CAS PubMed; (b) M. Fevre, J. Pinaud, Y. Gnanou, J. Vignolle and D. Taton, Chem. Soc. Rev., 2013, 42, 2142–2172 RSC; (c) E. Peris, Chem. Rev., 2018, 118, 9988–10031 CrossRef CAS PubMed; (d) D. Munz, Organometallics, 2018, 37, 275–289 CrossRef CAS; (e) R. S. Ghadwal, Synlett, 2019, 1765–1775 CAS.
- (a) W. A. Herrmann and B. Cornils, Angew. Chem., Int. Ed. Engl., 1997, 36, 2162 CrossRef CAS; (b) D. Bourissou, O. Guerret, F. P. Gabbai and G. Bertrand, Chem. Rev., 2000, 100, 39–92 CrossRef CAS PubMed; (c) Y. Ryu, G. Ahumada and C. W. Bielawski, Chem. Commun., 2019, 55, 4451–4466 RSC.
- (a) M. Poyatos, J. A. Mata and E. Peris, Chem. Rev., 2009, 109, 3677–3707 CrossRef CAS PubMed; (b) P. C. Purba, S. Bhattacharyya, M. Maity, S. Mukhopadhyay, P. Howlader and P. S. Mukherjee, Chem. Commun., 2019, 55, 8309–8312 RSC; (c) S. Gonell, E. Peris and M. Poyatos, Eur. J. Inorg. Chem., 2019, 3776–3781 CrossRef CAS.
- (a) W. A. Herrmann, L. J. Goossen, C. Köcher and G. R. J. Artus, Angew. Chem., Int. Ed. Engl., 1996, 35, 2805–2807 CrossRef CAS; (b) S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef CAS PubMed; (c) N. E. Kamber, W. Jeong, R. M. Waymouth, R. C. Pratt, B. G. G. Lohmeijer and J. L. Hedrick, Chem. Rev., 2007, 107, 5813–5840 CrossRef CAS PubMed.
- (a) K. M. Hindi, M. J. Panzner, C. A. Tessier, C. L. Cannon and W. J. Youngs, Chem. Rev., 2009, 109, 3859 CrossRef CAS PubMed; (b) L. Mercs and M. Albrecht, Chem. Soc. Rev., 2010, 39, 1903–1912 RSC; (c) L. Mercs, A. Neels, H. Stoeckli-Evans and M. Albrecht, Dalton Trans., 2009, 7168–7178 RSC.
- (a) A. J. Arduengo and L. I. Iconaru, Dalton Trans., 2009, 6903–6914 RSC; (b) S. Saravanakumar, M. K. Kindermann, J. Heinicke and M. Kockerling, Chem. Commun., 2006, 640–642 RSC.
- H. V. Huynh, Coord. Chem. Rev., 2018, 118, 9457–9492 CrossRef CAS PubMed.
- (a) M. D. Sanderson, J. W. Kamplain and C. W. Bielawski, J. Am. Chem. Soc., 2006, 128, 16514–16515 CrossRef CAS PubMed; (b) D. M. Khramov, V. M. Lynch and C. W. Bielawski, Organometallics, 2007, 26, 6042–6049 CrossRef CAS; (c) J. I. Bates and D. P. Gates, Organometallics, 2012, 31, 4529–4536 CrossRef CAS.
- D. Mendoza-Espinosa, B. Donnadieu and G. Bertrand, J. Am. Chem. Soc., 2010, 132, 7264–7265 CrossRef CAS PubMed.
- (a) K. R. Grundy and W. R. Roper, J. Organomet. Chem., 1975, 91, C61–C64 CrossRef CAS; (b) Z. Kelemen, O. Hollóczki, J. Oláh and L. Nyulászi, RSC Adv., 2013, 3, 7970–7978 RSC.
- D. Enders and T. Balensiefer, Acc. Chem. Res., 2004, 37, 534–541 CrossRef CAS PubMed.
- R. S. Ghadwal, H. W. Roesky, M. Granitzka and D. Stalke, J. Am. Chem. Soc., 2010, 132, 10018–10020 CrossRef CAS PubMed.
- K. E. Krahulic, G. D. Enright, M. Parvez and R. Roesler, J. Am. Chem. Soc., 2005, 127, 4142–4143 CrossRef CAS PubMed.
- J. I. Bates, P. Kennepohl and D. P. Gates, Angew. Chem., Int. Ed., 2009, 48, 9844–9847 CrossRef CAS PubMed.
- D. M. Khramov, A. J. Boydston and C. W. Bielawski, Angew. Chem., Int. Ed., 2006, 45, 6186–6189 CrossRef CAS PubMed.
- (a) E. Peris, Chem. Rev., 2018, 118, 9988–10031 CrossRef CAS PubMed; (b) M. Böhmer, G. Guisado-Barrios, F. Kampert, F. Roelfes, T. T. Yuan Tsai, E. Peris and E. Hahn, Organometallics, 2019, 38, 2120 CrossRef; (c) S. Gonell, R. G. Alabau, M. Poyatos and E. Peris, Chem. Commun., 2013, 49, 7126–7128 RSC.
- M. Schmidtendorf, C. Schulte to Brinke and F. E. Hahn, J. Organomet. Chem., 2014, 751, 620–627 CrossRef CAS.
- (a) P. K. Majhi, G. Schnakenburg and R. Streubel, Dalton Trans., 2014, 43, 16673–16679 RSC; (b) P. K. Majhi, G. Schnakenburg, Z. Kelemen, L. Nyulászi, D. P. Gates and R. Streubel, Angew. Chem., Int. Ed., 2013, 52, 10080–10083 CrossRef CAS PubMed; (c) D. Tapu, Z. McCarty and C. McMillen, Chem. Commun., 2014, 50, 4725–4728 RSC.
- (a) A. Koner, G. Pfeifer, Z. Kelemen, G. Schnakenburg, L. Nyulászi, T. Sasamori and R. Streubel, Angew. Chem., Int. Ed., 2017, 56, 9231–9235 CrossRef CAS PubMed; (b) A. Koner, Z. Kelemen, G. Schnakenburg, L. Nyulászi and R. Streubel, Chem. Commun., 2018, 54, 1182–1184 RSC.
- I. Begum, G. Schnakenburg and R. Streubel, Dalton Trans., 2016, 46, 2955–2962 RSC.
- N. C. Payne, A. Geissler, A. Button, A. R. Sasuclark, A. L. Schroll, E. L. Ruggles, V. N. Gladyshev and R. J. Hondal, Free Radicals Biol. Med., 2017, 104, 249–261 CrossRef CAS PubMed.
- A. G. Tennyson, R. J. Ono, T. W. Hudnall, D. M. Khramov, J. A. V. Er, J. W. Kamplain and C. W. Bielawski, Chem. – Eur. J., 2010, 16, 304–315 CrossRef CAS PubMed.
- 3′cis/trans, 7′cis/trans, 4a′ = calculated compounds with methyl substituents at N.
- L. Nyulászi, A. Forró and T. Veszprémi, Phys. Chem. Chem. Phys., 2000, 2, 3127–3129 RSC.
- K. Verlinden, H. Buhl, W. Frank and C. Ganter, Eur. J. Inorg. Chem., 2015, 2416–2425 CrossRef CAS.
- L. Nyulászi, O. Hollóczki, C. Lescop, M. Hissler and R. Réau, Org. Biomol. Chem., 2006, 4, 996–998 RSC.
- C. Hay, M. Hissler, C. Fischmeister, J. Rault-Berthelot, L. Toupet, L. Nyulászi and R. Reau, Chem. – Eur. J., 2001, 7, 4222–4236 CrossRef CAS.
- (a) M. Feroci, I. Chiarotto and A. Inesi, Catalysts, 2016, 6, 178 CrossRef; (b) M. Feroci, I. Chiarotto, F. D’Anna, F. Gala, R. Noto, L. Ornano, G. Zollo and A. Inesi, ChemElectroChem, 2016, 3, 1133–1141 CrossRef CAS.
- A. Doddi, M. Peters and M. Tamm, Chem. Rev., 2019, 119, 6994–7112 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1919566–1919569. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc08468a |
| This journal is © The Royal Society of Chemistry 2020 |