An activatable near-infrared fluorescent probe for methylglyoxal imaging in Alzheimer's disease mice†
Abstract
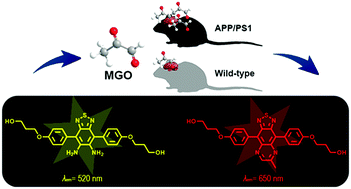
Visual detection of the methylglyoxal (MGO) level in the brain is critical for understanding its role in the onset and progression of AD. Herein, we disclosed a NIR fluorescent probe, DBTPP, for detecting MGO by utilizing a thiadiazole-fused o-phenylenediamine moiety as a MGO-specific sensing unit. DBTPP exhibits a series of distinct advantages, such as NIR emission, high selectivity and sensitivity, excellent acid-stability, and a huge off–on ratio. The probe could accurately monitor both exogenous and endogenous MGO variations in SH-SY5Y cells. Besides, it was able to image the endogenous MGO in a transgenic AD mouse model successfully, suggesting the great potential of MGO as a biomarker for early AD diagnosis.


 Please wait while we load your content...
Please wait while we load your content...