Facile synthesis of novel reduced graphene oxide@polystyrene nanospheres for sensitive label-free electrochemical immunoassay†
Abstract
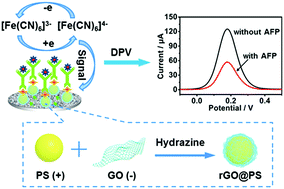
Herein, we report the synthesis of novel nanosized reduced graphene oxide@polystyrene nanospheres (rGO@PS NSs) through an electrostatic interaction method, and further their exploitation for highly sensitive label-free electrochemical immunoassay applications. These rGO@PS NSs provide a universal and promising carrier platform to develop excellent biosensors for clinical screening and diagnostic applications.


 Please wait while we load your content...
Please wait while we load your content...