Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
There is nothing wrong with being soft: using sulfur ligands to increase axiality in a Dy(III) single-ion magnet†
Angelos B.
Canaj
 a,
Sourav
Dey
b,
Oscar
Céspedes
c,
Claire
Wilson
a,
Sourav
Dey
b,
Oscar
Céspedes
c,
Claire
Wilson
 a,
Gopalan
Rajaraman
a,
Gopalan
Rajaraman
 *b and
Mark
Murrie
*b and
Mark
Murrie
 *a
*a
aSchool of Chemistry, University of Glasgow, University Avenue, Glasgow, G12 8QQ, UK. E-mail: mark.murrie@glasgow.ac.uk
bDepartment of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, Maharashtra 400076, India. E-mail: rajaraman@chem.iitb.ac.in
cSchool of Physics and Astronomy, University of Leeds, Leeds LS2 9JT, UK
First published on 26th December 2019
Abstract
A new air-stable sulfur-ligated Dy(III) single-ion magnet has been successfully isolated with Ueff = 638 K and hysteresis loops open up to 7 K. In silico studies show that the S co-ligands significantly boost the axiality and that Te- and Se-donors have the potential to further enhance the magnetic properties.
The interest in Single-Molecule Magnets (SMMs), i.e. molecular systems, which display the ability to block magnetisation, resulting in the appearance of magnetic hysteresis of molecular origin,1 is because these systems could revolutionise electron spin-based technologies.2 Lanthanide SMMs are often associated with large magnetic moments and large magnetic anisotropy, with Dy(III) being a key component of single-ion magnets (SIMs).3 Through the combination of theory4 and experiment, an exciting era has emerged with a new generation of SMMs/SIMs showing impressive energy barriers5 and high blocking temperatures, TB,6 reaching 80 K.7 In fact, complexes with D3h,8D4d9 and D5h,10 axial point group symmetries are very promising. Our group recently reported the blueprint for generating strong uniaxial magnetic anisotropy for Dy(III) in ∼D6h symmetry (hexagonal bipyramidal), by combining a strong linear axial ligand field with a weak equatorial ligand field, by using a polydentate ligand LN6 (LN6 = N6-hexagonal plane from the neutral Schiff base ligand formed from 2,6-diacetylpyridine and ethylenediamine).11
Most of the reported 4f-SIMs incorporate traditional ligands with O-, N-, C- and halogen-donor atoms.3 Rarer are the examples of SMMs exploring ligands with more “exotic” donor atoms from the main group, as was reviewed recently by Guo et al.12 Recently we have explored how ligand electronics can tune SIM properties13 and herein, we sought to explore how different donor groups originating from the p block affect the magnetisation dynamics. We report for the first time the synthesis, structure, magnetic characterisation and ab initio studies of [DyIIILON3(C5H10NS2)2]·0.5THF (1) (Fig. 1) which is a S-ligated single-ion magnet with hysteresis loops open up to 7 K and a magnetisation reversal barrier of Ueff = 638 K, which is unprecedented in the very small family of S-ligated Dy(III) single-ion magnets (see Table 1).14–20 Compound 1 was isolated by using the cage-like ligand N-(3,5-di-tert-butyl-2-hydroxybenzyl)-N,N-bis(2-pyridylmethyl)amine (HLON3) (see ESI†).21
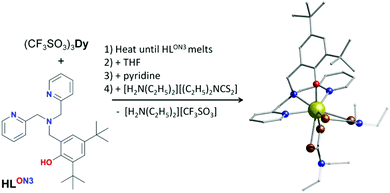 | ||
| Fig. 1 Synthesis and structure of 1. Dy, gold; O, red; N, blue; S, brown; C, grey. H atoms and solvent are omitted for clarity. | ||
| Complex | U eff (K) | τ 0 (s) | H dc (Oe) | Ref. |
|---|---|---|---|---|
| [DyIII(Pc)(STBPP)] | 194 | 4.7 × 10−8 | 0 | 14 |
| [(dtc)3Dy(dmbipy)] | 9.8 | 1.9 × 10−6 | 1000 | 15 |
| [(dtc)3Dy(phen)] | 20 | 1.80 × 10−8 | 400 | 16 |
| [(dbm)2Dy(dtc)(phen)] | 45 | 2.8 × 10−8 | 1000 | 16 |
| 40 | 4.1 × 10−8 | 0 | ||
| [Dy((−)-pbipy)(pdtc)3] | 56.7 | 4.2 × 10−7 | 3000 | 17 |
| Dy2S@C82-C3v | 6.5 | 3.6 | 18 | |
| 48 | 6.2 × 10−4 | |||
| 1232 | 6.0 × 10−13 | |||
| [Li(thf)4][Dy4{N(SiMe3)2}4(μ-SEt)8(μ4-SEt)] | 66 | 4.3 × 10−6 | 0 | 19 |
| 71 | 2.1 × 10−6 | 2000 | ||
| [{Cp2′Dy(μ-SSiPh3)}2] | 192 | 2.38 × 10−7 | 0 | 20 |
| [Dy III L ON3 (C 5 H 10 NS 2 ) 2 ] | 638 | 2.99 × 10 −12 | 0 | This work |
Our synthetic strategy generates one short Dy–O bond, to direct the magnetic anisotropy,22 and three longer Dy–N bonds.23 The rest of the coordination sphere is completed with soft S-donor groups, by using diethyldithiocarbamate co-ligands, (Fig. 1) giving longer Dy–S bonds (Fig. S1 and Table S2, ESI†). Importantly, through a detailed in silico study we also examine how O-, Se- and Te-based co-ligands affect the calculated magnetisation reversal barrier, Ucal, of 1.
Complex 1 (Fig. 1) was isolated from a dry THF solution (see ESI†) with the phase purity of the bulk sample confirmed by powder X-ray diffraction (Fig. S2, ESI†). Continuous shape measures24 analysis gives a value of 2.7 for a biaugmented trigonal prism geometry (C2v symmetry) (Fig. S3 and Table S3, ESI†). The only strong oxygen donor group in 1 has a relatively short Dy–O bond length (2.1591(16) Å) while the Dy–N and Dy–S bonds fall in the range of 2.5237(18)–2.5711(17) Å and 2.8133(5)–2.9647(6) Å, respectively (Table S2 and Fig. S1, ESI†). Additionally, there is no intermolecular hydrogen bonding in 1, while the shortest Dy⋯Dy distance is 8.39 Å (Fig. S4, ESI†).
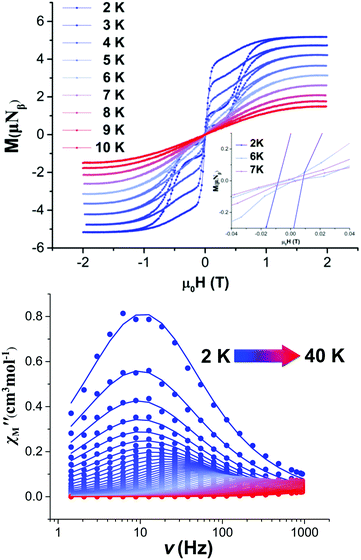
The dc magnetic susceptibility and magnetisation measurements for 1 are shown in Fig. S5 (ESI†). The field cooled (FC) and zero-field cooled (ZFC) magnetic susceptibility show divergence at 5 K (Fig. S6, ESI†) with the magnetic hysteresis measurements, M(H) loops, performed on a powder sample of 1, remaining open up to 7 K (average sweep rate of 20 mT s−1) (Fig. 2 upper and Fig. S7, ESI†).
Measurements of the variable temperature alternating current (ac) susceptibility between 1–940 Hz were performed in order to investigate the dynamics of the magnetisation for 1 (Fig. 2 lower and Fig. S8–S16, ESI†). Under zero external dc field, the out-of-phase, χM′′ magnetic susceptibility data exhibit well-defined maxima with χM′′ peaks clearly observable up to ∼35 K (Fig. S8, ESI†), indicating a high magnetisation reversal barrier. The relaxation times, τ, were extracted from the fits of the Argand plots of χM′′ vs. χM′ using the generalized Debye model (Fig. S11, ESI†).25 The α parameters found are in the range of 0–0.3 (2–40 K) for 1. The τ−1vs. T data were fitted using the equation τ−1 = τQTM−1 + CTn + τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/T), in which C and n are parameters of the Raman process and τQTM is the rate of the quantum tunnelling of magnetisation (QTM).26 The best fit gives a magnetisation reversal barrier, Ueff of 638 K, τ0 = 2.99 × 10−12 s, n = 3.24, C = 0.02 K−n s−1, τQτM = 0.017 s, under zero dc field (Fig. S17, ESI†) and Ueff = 656 K, τ0 = 1.94 × 10−12 s, n = 3.96 and C = 3.95 × 10−5 K−n s−1 under an optimum field of 1200 Oe (Fig. S18, ESI†). The observed values of the pre-factor τ0,10C and n are within the commonly observed range for Dy(III) SMMs.3 The exponent n of the Raman process has a smaller value than expected for a Kramers ion (n = 9) suggesting the presence of Raman processes involving optical acoustic phonons.26 To the best of our knowledge, this is the largest magnetisation reversal barrier observed for a Dy(III) single-ion magnet that has S-donor ligands (see Table 1).
exp(−Ueff/T), in which C and n are parameters of the Raman process and τQTM is the rate of the quantum tunnelling of magnetisation (QTM).26 The best fit gives a magnetisation reversal barrier, Ueff of 638 K, τ0 = 2.99 × 10−12 s, n = 3.24, C = 0.02 K−n s−1, τQτM = 0.017 s, under zero dc field (Fig. S17, ESI†) and Ueff = 656 K, τ0 = 1.94 × 10−12 s, n = 3.96 and C = 3.95 × 10−5 K−n s−1 under an optimum field of 1200 Oe (Fig. S18, ESI†). The observed values of the pre-factor τ0,10C and n are within the commonly observed range for Dy(III) SMMs.3 The exponent n of the Raman process has a smaller value than expected for a Kramers ion (n = 9) suggesting the presence of Raman processes involving optical acoustic phonons.26 To the best of our knowledge, this is the largest magnetisation reversal barrier observed for a Dy(III) single-ion magnet that has S-donor ligands (see Table 1).
In order to gain insight into the mechanism that governs the magnetic relaxation of 1, we have performed ab initio calculations using the CASSCF/RASSI-SO/SINGLE_ANISO approach implemented in MOLCAS 8.227 (see ESI†). The eight Kramers Doublets (KDs) in 1 span an energy range of 964 K (Table S4, ESI†). The ground state (mJ = ±15/2) of the Dy(III) ion in 1 is highly anisotropic with near-perfect axiality (gzz = 19.859, gxx = gyy = 0.001, Table S4, ESI†). The main anisotropy axis is nearly collinear with the relatively short Dy–O bond (Fig. S19, ESI†) resulting from our synthetic strategy. Using the CASSCF wavefunction, the computed Loprop28 charge on the oxygen atom is found to be nearly three times larger than the nitrogen atoms of the LON3 ligand and twice as large as the sulfur atoms of the diethyldithiocarbamate ligands (Fig. S20, ESI†). The axial nature is also observed for the first and second excited states (mJ = ±13/2, gxx = 0.023, gyy = 0.028, gzz = 17.359 and mJ = ± 11/2, gxx = 0.281 gyy = 0.380, gzz = 14.372, Table S4, ESI†), with the higher KDs showing relatively stronger admixtures (Fig. 3). The maximum calculated relaxation barrier, Ucal, is estimated at 651 K (Fig. 3), which is in excellent agreement with the experimentally determined magnetisation reversal barrier (Ueff) of 638 K found in zero applied dc field. A relatively small transverse magnetic moment is calculated for the first three KDs (0.35 × 10−3, 0.88 × 10−2, 1.2 × 10−1μB, respectively), which indicates relaxation via the third excited state (Fig. 3). In addition, the Orbach processes for the mJ and mJ + 1 excited states of opposite magnetisation between the first four KDs are found to be very small (≤0.43 μB, Fig. 3).
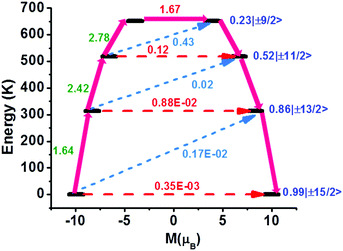 | ||
| Fig. 3 Ab initio calculated relaxation dynamics for 1. The arrows show the connected energy states with the number representing the matrix element of the transverse moment (see text for details). The black line indicates the KDs as a function of magnetic moments. The red dashed arrow represents QTM (QTM = quantum tunnelling of the magnetisation) via the ground state and TA-QTM (TA-QTM = thermally assisted QTM) via excited states. The blue dashed arrow indicates possible Orbach processes. The pink thick arrow indicates the mechanism of magnetic relaxation. The numbers above each arrow represent corresponding transverse matrix elements for the transition magnetic moments.29 | ||
To investigate the importance of the coordination environment and the ligand electronics on the magnetisation dynamics of 1, we have changed the co-ligand coordination environment in silico. We have created a family of three different model systems and used ab initio calculations to examine how O-, Te- and Se-based co-ligands affect the calculated magnetisation reversal barrier of 1 (Fig. 4 and Fig. S21, S22, ESI†). Importantly, replacing the S-atoms of the diethyldithiocarbamate co-ligands with more commonly used oxygen donors (i.e. common carboxylate ligands, model 1-O, Fig. S22 upper, ESI†) gives stronger transverse components, with larger gxx/gyy values obtained for the ground and excited states (see Table S5 (ESI†) for model 1-O). Specifically, the QTM probabilities are calculated to be larger for the first three KDs of model 1-O (0.63 × 10−3, 0.31 × 10−1 and 0.85 μB see Fig. S21 upper, ESI†) compared to 1 (Fig. 3), leading to a smaller calculated barrier of Ucal = 528 K (see Fig. 4 and Fig. S21, Table S5, ESI†). These results are similar to earlier observations for Dy–O vs. Dy–S substitution14 suggesting a likely generality of such behaviour in Dy(III) complexes.
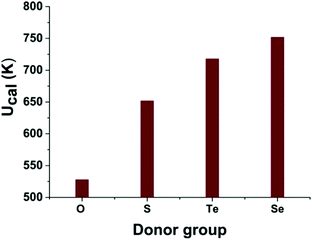 | ||
| Fig. 4 The effect of the O-, S-, Te- and Se-donor co-ligands on the Ucal barrier using in silico models based on 1. | ||
In contrast, the gxx/gyy values obtained for model systems 1-Te and 1-Se, where the S-atoms in 1 are replaced with Te- and Se-atoms (Fig. S22 lower, ESI†), suggest that the magnetisation relaxes via the fourth KD, giving higher calculated barriers of Ucal = 718 K for 1-Te and 752 K for 1-Se (see Fig. 4 and Fig. S21, Table S5, ESI†). Importantly, our results suggest that substitution of the S-atoms in 1 with O-atoms favours a stronger transverse anisotropy, while substitution with Te- and/or Se-atoms stabilises stronger axiality, with smaller transverse magnetic moments calculated for the first four KDs and smaller gxx/gyy values (see Fig. 4 and Fig. S21, Table S5, ESI†). This is in excellent agreement with a study performed on pnictogen-ligated compounds.30 The ratio between B02 and the corresponding non-axial crystal field parameters increases in the following order 1-O < 1 < 1-Te < 1-Se (Table S7, ESI†) in line with the increasing Ucal barrier (Fig. 4).
In conclusion, [DyIIILON3(C5H10NS2)2]·0.5THF (1) is the first S-ligated single-ion magnet with hysteresis loops open up to 7 K and a magnetisation reversal barrier of 638 K, which is significantly higher than any reported Dy(III) SIM that has S-donor ligands (Table 1). This novel complex was isolated by a carefully designed synthetic strategy that generates one short Dy–O bond,22 which directs the magnetic anisotropy, combined with three longer Dy–N bonds, with the remainder of the coordination sphere completed with soft S-donor groups, giving longer Dy–S bonds. Furthermore, through detailed in silico studies we examine how O-, Se- and Te-based co-ligands affect the calculated magnetisation reversal barrier and magnetisation dynamics in 1, finding higher Ucal values for Te- and Se-based co-ligands. We hope that this study will generate further interest in the investigation of S-ligated SIMs and prompt the study of new Te- and Se-ligated SIMs.
We thank EPSRC UK (EP/N01331X/1, EP/M000923/1); UGC (SRF fellowship); UGC-UKIERI (184-1/2018(lC)); UGC and SERB (CRG/2018/000430); CSIR (01(2980)/19/EMR-II) and IIT Bombay (CRAY supercomputing facility) for funding.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. M. J. Aubin, M. W. Wemple, D. M. Adams, H.-L. Tsai, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1996, 118, 7746 CrossRef CAS; R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef; A. Caneschi, D. Gatteschi, R. Sessoli and M. A. Novak, Nature, 1993, 365, 141 CrossRef; C. J. Milios, A. Vinslava, W. Wernsdorfer, S. Moggach, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 2754 CrossRef PubMed.
- E. M. Pineda, C. Godfrin, F. Balestro, W. Wernsdorfer and M. Ruben, Chem. Soc. Rev., 2018, 47, 501 RSC; R. Vincent, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Nature, 2012, 488, 357 CrossRef CAS PubMed; M. Shiddiq, D. Komijani, Y. Duan, A. Gaita-Ariño, E. Coronado and S. Hill, Nature, 2016, 531, 348 CrossRef PubMed; C. Godfrin, A. Ferhat, R. Ballou, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Phys. Rev. Lett., 2017, 119, 187702 CrossRef PubMed; A. Gaita-Ariño, F. Luis, S. Hill and E. Coronado, Nat. Chem., 2019, 11, 301 CrossRef PubMed; M. Atzori and R. Sessoli, J. Am. Chem. Soc., 2019, 141, 11339 CrossRef PubMed.
- M. Heras Ojea, L. H. C. Maddock and R. A. Layfield, Lanthanide Organometallics as Single-Molecule Magnets, in Topics in Organometallic Chemistry, Springer, Berlin, Heidelberg, 2019 CrossRef CAS PubMed; F.-S. Guo and R. A. Layfield, Acc. Chem. Res., 2018, 51, 1880 CrossRef CAS PubMed; M. Feng and M.-L. Tong, Chem. – Eur. J., 2018, 24, 7574 CrossRef PubMed; A. K. Bar, P. Kalita, M. K. Singh, G. Rajaraman and V. Chandrasekhar, Coord. Chem. Rev., 2018, 367, 163 CrossRef; S. G. McAdams, A.-M. Ariciu, A. K. Kostopoulos, J. P. S. Walsh and F. Tuna, Coord. Chem. Rev., 2017, 346, 216 CrossRef; Z. Zhu, M. Guo, X.-L. Li and J. Tang, Coord. Chem. Rev., 2019, 378, 350 CrossRef; L. Spree and A. A. Popov, Dalton Trans., 2019, 48, 2861 RSC.
- L. Ungur and L. F. Chibotaru, Inorg. Chem., 2016, 55, 10043 CrossRef CAS PubMed; J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC; N. F. Chilton, Inorg. Chem., 2015, 54, 2097 CrossRef PubMed; A. Lunghi, F. Totti, R. Sessoli and S. Sanvito, Nat. Commun., 2017, 8, 14620 CrossRef PubMed.
- S.-D. Jiang, B.-W. Wang, H.-L. Sun, Z.-M. Wang and S. Gao, J. Am. Chem. Soc., 2011, 133, 4730 CrossRef CAS PubMed; Y.-S. Meng, L. Xu, J. Xiong, Q. Yuan, T. Liu, B.-W. Wang and S. Gao, Angew. Chem., Int. Ed., 2018, 57, 1 CrossRef; Y.-S. Ding, K.-X. Yu, D. Reta, F. Ortu, R. E. P. Winpenny, Y.-Z. Zheng and N. F. Chilton, Nat. Commun., 2018, 9, 3134 CrossRef PubMed; S. Demir, M. I. Gonzalez, L. E. Darago, W. J. Evans and J. R. Long, Nat. Commun., 2017, 8, 2144 CrossRef PubMed; P. E. Kazin, M. A. Zykin, V. V. Utochnikova, O. V. Magdysyuk, A. V. Vasiliev, Y. V. Zubavichus, W. Schnelle, C. Felser and M. Jansen, Angew. Chem., Int. Ed., 2017, 56, 1 CrossRef PubMed; F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Buchner and A. A. Popov, Nat. Commun., 2017, 8, 16098 CrossRef PubMed.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamaki and R. A. Layfield, Angew. Chem., Int. Ed., 2017, 56, 11445 CrossRef CAS PubMed; A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439 CrossRef PubMed; K. R. McClain, C. A. Gould, K. Chakarawet, S. J. Teat, T. J. Groshens, J. R. Long and B. G. Harvey, Chem. Sci., 2018, 9, 8492 RSC.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400 CrossRef CAS PubMed.
- P. Zhang, L. Zhang, C. Wang, S. Xue, S.-Y. Lin and J. Tang, J. Am. Chem. Soc., 2014, 136, 4484 CrossRef CAS PubMed; K. L. M. Harriman, J. L. Brosmer, L. Ungur, P. L. Diaconescu and M. Murugesu, J. Am. Chem. Soc., 2017, 139, 1420 CrossRef PubMed.
- J. Wu, J. Jung, P. Zhang, H. Zhang, J. Tang and B. Le Guennic, Chem. Sci., 2016, 7, 3632 RSC; K. Katoh, S. Yamashita, N. Yasuda, Y. Kitagawa, B. K. Breedlove, Y. Nakazawa and M. Yamashita, Angew. Chem., Int. Ed., 2018, 57, 9262 CrossRef CAS PubMed; S. Bala, G.-Z. Huang, Z.-Y. Ruan, S.-G. Wu, Y. Liu, L.-F. Wang, J.-L. Liu and M.-L. Tong, Chem. Commun., 2019, 55, 9939 RSC.
- Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829 CrossRef CAS PubMed; Y.-S. Ding, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 16071 CrossRef PubMed; J. Liu, Y.-C. Chen, J.-L. Liu, V. Vieru, L. Ungur, J.-H. Jia, L. F. Chibotaru, Y. Lan, W. Wernsdorfer, S. Gao, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 5441 CrossRef PubMed; S. K. Gupta, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Chem. Sci., 2016, 7, 5181 RSC; J. L. Liu, Y. C. Chen, Y. Z. Zheng, W. Q. Lin, L. Ungur, W. Wernsdorfer, L. F. Chibotaru and M. L. Tong, Chem. Sci., 2013, 4, 3310 RSC; A. B. Canaj, M. K. Singh, C. Wilson, G. Rajaraman and M. Murrie, Chem. Commun., 2018, 54, 8273 RSC.
- A. B. Canaj, S. Dey, E. R. Marti, C. Wilson, G. Rajaraman and M. Murrie, Angew. Chem., Int. Ed., 2019, 58, 14146 CrossRef CAS PubMed; A. B. Canaj, S. Dey, E. R. Marti, C. Wilson, G. Rajaraman and M. Murrie, Angew. Chem., 2019, 131, 14284 CrossRef.
- F.-S. Guo, A. K. Bar and R. A. Layfield, Chem. Rev., 2019, 119, 8479 CrossRef CAS PubMed.
- A. B. Canaj, M. K. Singh, E. R. Marti, M. Damjanović, C. Wilson, O. Céspedes, W. Wernsdorfer, G. Rajaraman and M. Murrie, Chem. Commun., 2019, 55, 5950 RSC.
- W. Cao, C. Gao, Y.-Q. Zhang, D. Qi, T. Liu, K. Wang, C. Duan, S. Gao and J. Jiang, Chem. Sci., 2015, 6, 5947 RSC.
- S.-S. Liu, J.-M. Zhao, S. Deng, J. Xiong, M.-F. Yang, J.-X. Li, S.-J. Lin, Y.-Q. Zhang and B.-W. Wang, Inorg. Chem. Commun., 2018, 95, 82 CrossRef CAS.
- S.-S. Liu, K. Lang, Y.-Q. Zhang, Q. Yang, B.-W. Wang and S. Gao, Dalton Trans., 2016, 45, 8149 RSC.
- H.-R. Wen, K. Yang, S.-J. Liu, F.-Y. Liang, X.-R. Xie, C.-M. Liu and Y.-W. Li, Inorg. Chim. Acta, 2018, 473, 145 CrossRef CAS.
- C.-H. Chen, D. S. Krylov, S. M. Avdoshenko, F. Liu, L. Spree, R. Yadav, A. Alvertis, L. Hozoi, K. Nenkov, A. Kostanyan, T. Greber, A. Wolter-Giraud and A. A. Popov, Chem. Sci., 2017, 8, 6451 RSC.
- D. N. Woodruff, F. Tuna, M. Bodensteiner, R. E. P. Winpenny and R. A. Layfield, Organometallics, 2013, 32, 1224 CrossRef CAS.
- F. Tuna, C. A. Smith, M. Bodensteiner, L. Ungur, L. F. Chibotaru, E. J. L. McInnes, R. E. P. Winpenny, D. Collison and R. A. Layfield, Angew. Chem., Int. Ed., 2012, 51, 6976 CrossRef CAS PubMed.
- S. Zheng, T. C. Berto, E. W. Dahl, M. B. Hoffman, A. L. Speelman and N. Lehnert, J. Am. Chem. Soc., 2013, 135, 4902 CrossRef CAS PubMed.
- L. Ungur and L. F. Chibotaru, Phys. Chem. Chem. Phys., 2011, 13, 20086 RSC.
- S. K. Singh, T. Gupta and G. Rajaraman, Inorg. Chem., 2014, 53, 10835 CrossRef CAS PubMed.
- M. Pinskya and D. Avnir, Inorg. Chem., 1998, 37, 5575 CrossRef CAS PubMed; D. Casanova, M. Llunell, P. Alemany and S. Alvarez, Chem. – Eur. J., 2005, 11, 1479 CrossRef PubMed.
- D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford Univ. Press, Yton, 2006 Search PubMed.
- K. N. Shrivastava, Phys. Status Solidi B, 1983, 117, 437 CrossRef CAS; S. Q. Wu, Y. Miyazaki, M. Nakano, S. Q. Su, Z. S. Yao, H. Z. Kou and O. Sato, Chem. – Eur. J., 2017, 23, 10028 CrossRef PubMed; A. Singh and K. N. Shrivastava, Phys. Status Solidi B, 1979, 95, 273 CrossRef.
- F. Aquilante, J. Autschbach, R. K. Carlson, L. F. Chibotaru, M. G. Delcey, L. De Vico, I. Fdez Galvan, N. Ferre, L. M. Frutos and L. Gagliardi, et al. , J. Comput. Chem., 2016, 37, 506 CrossRef CAS PubMed; L. F. Chibotarua and L. Ungur, J. Chem. Phys., 2012, 137, 064112 CrossRef PubMed; A. A. Granovsky, J. Chem. Phys., 2011, 134, 214113 CrossRef PubMed.
- L. Gagliardi, R. Lindh and G. Karlstrom, J. Chem. Phys., 2004, 121, 4494 CrossRef CAS PubMed.
- L. Ungur, M. Thewissen, J. P. Costes, W. Wernsdorfer and L. F. Chibotaru, Inorg. Chem., 2013, 52, 6328 CrossRef CAS PubMed.
- T. Pugh, F. Tuna, L. Ungur, D. Collison, E. J. L. McInnes, L. F. Chibotaru and R. A. Layfield, Nat. Commun., 2015, 6, 7492 CrossRef CAS PubMed; T. Pugh, V. Vieru, L. F. Chibotaru and R. A. Layfield, Chem. Sci., 2016, 7, 2128 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, magnetic studies, crystallographic details, ab initio studies. CCDC 1950766. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc07292f |
| This journal is © The Royal Society of Chemistry 2020 |