Immunostimulatory biomaterials to boost tumor immunogenicity
Oluwaseyi T.
Shofolawe-Bakare
a,
Larry D.
Stokes
b,
Mehjabeen
Hossain
c,
Adam E.
Smith
ab and
Thomas A.
Werfel
 *abc
*abc
aDepartment of Chemical Engineering, University of Mississippi, University, MS, USA. E-mail: tawerfel@olemiss.edu
bDepartment of Biomedical Engineering, University of Mississippi, University, MS, USA
cDepartment of BioMolecular Sciences, University of Mississippi, University, MS, USA
First published on 24th August 2020
Abstract
Cancer immunotherapy is exhibiting great promise as a new therapeutic modality for cancer treatment. However, immunotherapies are limited by the inability of some tumors to provoke an immune response. These tumors with a ‘cold’ immunological phenotype are characterized by low numbers of tumor-infiltrating lymphocytes, high numbers of immunosuppressive leukocytes (e.g. regulatory T cells, tumor-associated macrophages), and high production of immune-dampening signals (e.g. IL-10, TGF-β, IDO-1). Strategies to boost the aptitude of tumors to initiate an immune response (i.e. boost tumor immunogenicity) will turn ‘cold’ tumors ‘hot’ and augment the anti-tumor efficacy of current immunotherapies. Approaches to boost tumor immunogenicity already show promise; however, multifaceted delivery and immunobiology challenges exist. For instance, systemic delivery of many immune-stimulating agents causes off-target toxicity and/or the development of autoimmunity, limiting the administrable dose below the threshold needed to achieve efficacy. Moreover, once administered in vivo, molecules such as the nucleic acid-based agonists for many pattern recognition receptors are either rapidly cleared or degraded, and don't efficiently traffic to the intracellular compartments where the receptors are located. Thus, these nucleic acid-based drugs are ineffective without a delivery system. Biomaterials-based approaches aim to enhance current strategies to boost tumor immunogenicity, enable novel strategies, and spare dose-limiting toxicities. Here, we review recent progress to improve cancer immunotherapies by boosting immunogenicity within tumors using immunostimulatory biomaterials.
1. Introduction
1.1 Cancer immunotherapy
Cancer immunotherapy harnesses the innate capabilities of the body's immune system to abate growth and metastasis of tumors. Immunotherapies have shown tremendous potential as a means of treating a broad range of malignancies and over the past decade there has been a significant proliferation in the amount of research conducted using immunotherapies to treat cancers.1 Approaches that harness the immune system to treat cancer include cancer vaccines, adoptive T cell therapy (ACT), immune checkpoint blockade (ICB), and others.Breakthroughs in ICB therapy have revolutionized cancer treatment, particularly difficult-to-treat and metastatic malignancies.2 Indeed, new food and drug administration (FDA) approvals to expand the usage of ICBs are announced regularly. ICB therapy targets T cells that possess co-inhibitory molecules, or “checkpoints”. Cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are the most widely studied checkpoints in cancer immunotherapy.3 In normal physiology, these checkpoints prevent overstimulation of the immune system, maintain peripheral self-tolerance, and facilitate resolution of immune responses. Unfortunately, tumors can exploit the immune checkpoints to suppress immunity inside the tumor microenvironment and evade elimination by the immune system (i.e. immunoediting). For instance, tumor cells can upregulate checkpoint molecules, e.g. PD-L1 (programmed death ligand-1), the ligand for PD-1, to promote peripheral T cell exhaustion.4 Antagonistic antibodies blocking immune checkpoints promote anti-tumor immune responses.3 Ipilimumab (CTLA-4 antagonist) was the first FDA-approved drug in this category.5 Pembrolizumab and nivolumab (both PD-1 antagonists) are currently approved for several different indications,6 including second-line therapy for renal cell cancer (nivolumab) and metastatic non-small cell lung cancer (pembrolizumab).6
Cancer vaccines support presentation of tumor antigens to T cells for the prevention and treatment of cancer. Cancer vaccines are used in two ways: as a preventative medicine (prophylactically) or as a treatment for existing malignancy (therapeutically).7 Cancer vaccines such as those containing viral antigens have been developed for prophylactic administration to prevent tumorigenesis. For example, human papilloma virus (HPV) vaccines reduce the risk of cervical cancer8 and hepatitis B virus vaccines help to prevent hepatocellular carcinoma in high risk populations.9 There are currently three types of therapeutic vaccines being investigated: dendritic cell vaccines (DC vaccines), peptide vaccines, and genetic vaccines.7 Dendritic cells perform their function as antigen-presenting cells (APCs) by engulfing protein antigens, degrading the antigens, and presenting peptides to T cells via either major histocompatibility complex (MHC) class-I or class-II.10 Peptides show promise for rapid development of personalized vaccines at reduced cost compared to DC therapy, and multiple peptides can be combined to produce a more effective vaccine than single peptides, as a single tumor-associated antigen might be edited or presented in different stages of cancer progression.11 Genetic vaccines use DNA plasmids or other gene constructs such as mRNA to directly transfer the coding segment of antigens or antigen fragments to APCs.7 For instance, a self-adjuvanted RNA vaccine for prostate cancer CAV9103 is currently in phase IIb clinical trials.12
ACT is an immunotherapy approach that uses an expanded pool of natural or genetically-modified T cells with improved ability to recognize and infiltrate tumors.13 Presently, three types of ACTs are being developed as cancer therapeutics: tumor-infiltrating lymphocytes (TILs), T cell receptor (TCR) T cells, and CAR (chimeric antigen receptor) T cells.14 TILs are in vitro expanded T cells which upon infusion into patients’ blood induce overall response rates (ORRs) of >50% in melanoma patients.15 TCR therapy is a redirected therapy where T cells are engineered to recognize a specific HLA-peptide complex.16 For this purpose, tumor-specific antigen NY-ESO-1 or MART-1 have been used and shown to achieve durable responses in sarcoma and metastatic melanoma patients.15 Another redirected T cell therapy is CAR T cell therapy. These engineered T cells are transduced with a chimera construct containing an extracellular domain, a single-chain variable fragment of light and heavy chain (scFv) antibody against specific extracellular receptors on tumor cells, and an intracellular domain to activate T cells via CD3 and co-stimulatory receptors CD-28 and 4-1BB.16
Critical for the success of all immunotherapies is the inherent immunogenicity of a tumor, or otherwise, the ability to ‘artificially’ stimulate immunogenicity within the tumor microenvironment.
1.2 Tumor immunogenicity
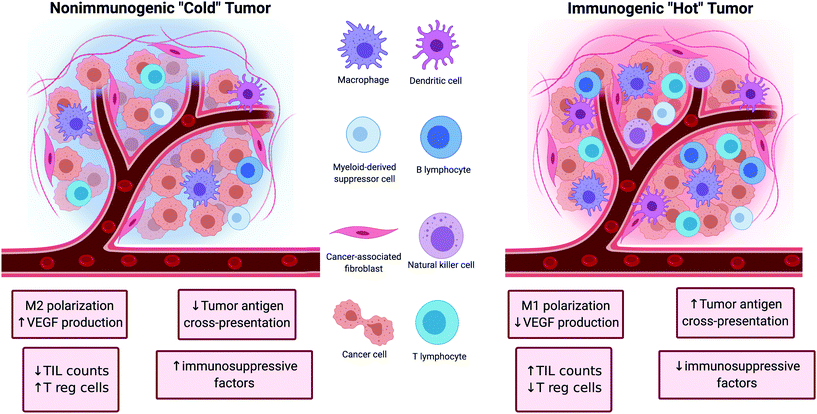
Tumors can be broadly classified into two major categories depending on their ability to initiate an immune response (Fig. 1). The first category of tumors is characterized by high TIL counts, high antigen cross-presentation, low immune checkpoint expression, and low presence of immunosuppressive factors. These tumors are referred to as ‘hot’ because of their inherent immunogenicity and high amounts of immune activity. The other category of tumors is characterized by the opposite features (low TIL counts, low antigen cross-presentation, high immune checkpoint expression, and high amounts of immunosuppressive factors). These tumors are referred to as ‘cold’ tumors because of their low immune activity. The nature of the tumor plays a significant role in determining the efficacy of cancer immunotherapies as ‘hot’ tumors tend to correlate with better clinical responses to treatment than ‘cold’ tumors.17The tumor microenvironment has many immunosuppressive pathways that hinder the efficacy of immune cells to mediate anti-tumor immunity. For instance, Bonaventura et al. identified four factors that determine the level of T cell infiltration in tumors: presence of tumor antigens, presence of and antigen presentation by innate immune cells such as dendritic cells (DCs), the down regulation of chemokines that attract DCs and T cells, and the secretion of immunosuppressive factors in the tumor microenvironment.18 These, and other pathways presented below, provide a number of actionable molecular targets that could be modulated to reverse immunosuppression and boost tumor immunogenicity. These efforts aim to shift tumors from a ‘cold’, immunosuppressive phenotype to ‘hot’, immune-active phenotype and increase the rate of response to immunotherapies such as immune checkpoint blockade and adoptive T cell transfer. Improved immunotherapies of the future will likely include more thorough consideration of immunogenicity in the tumor microenvironment and employ multiple strategies to prime the microenvironment prior to and in combination with immunotherapy.
Tregs can induce an immunosuppressive microenvironment within the tumor by releasing anti-inflammatory cytokines such as transformative growth factor-beta (TGF-β) and IL-10.19,21 These cytokines are known to inhibit several APC and CTL functions such as proliferation, differentiation, inflammatory cytokine production, expression of co-stimulatory molecules, and cytotoxicity.23–26 For example, Larmonier et al.26 showed that Tregs obtained from tumor-bearing mice hamper the activity of DCs by releasing TGF-β and IL-10. Also, Chen et al.27 showed that antigen-specific CTLs that have a dominant-negative TGF-β receptor maintained similar ability to reject tumors in CT44 murine colon cancer models in the presence or absence of Tregs while CTLs without the dominant-negative TGF-β receptor could not reject tumors. Another mechanism by which Tregs suppress the immune system is through the expression of CTLA-4. CTLA-4 and the T cell co-stimulatory molecule CD28 share the same ligands (CD80 and CD86), and CTLA-4 can block binding of CD28 to these ligands, resulting in impaired co-stimulation of T cells.28,29 Other immunosuppressive mechanisms such as the secretion of granzyme B,30–32 cytokine deprivation,19,21,33 and the inhibition of the phosphatase and tensin homolog (PTEN) pathway34,35 are also attributed to Tregs.
Like Tregs, tumor-associated macrophages (TAMs) and other myeloid-derived suppressor cells (MDSCs) support immunosuppression via multifaceted roles within the tumor microenvironment and are broadly considered pro-tumorogenic (Fig. 2). TAMs are some of the most prevalent cells within many tumors and are known to support growth and metastasis of advanced malignancies. TAMs secrete pro-angiogenic factors like vascular endothelial growth factor (VEGF), and promote the “angiogenic switch”, a process which provides tumors with growth advantages and aides in the transition to malignancy.36,37 They also release matrix metalloproteinases and chemokines like CCL18 which promote tumor invasion and metastasis.38,39 Furthermore, they release anti-inflammatory cytokines like IL-10 and express PD-L1 which can suppress CTL-specific anti-tumor immunity.40
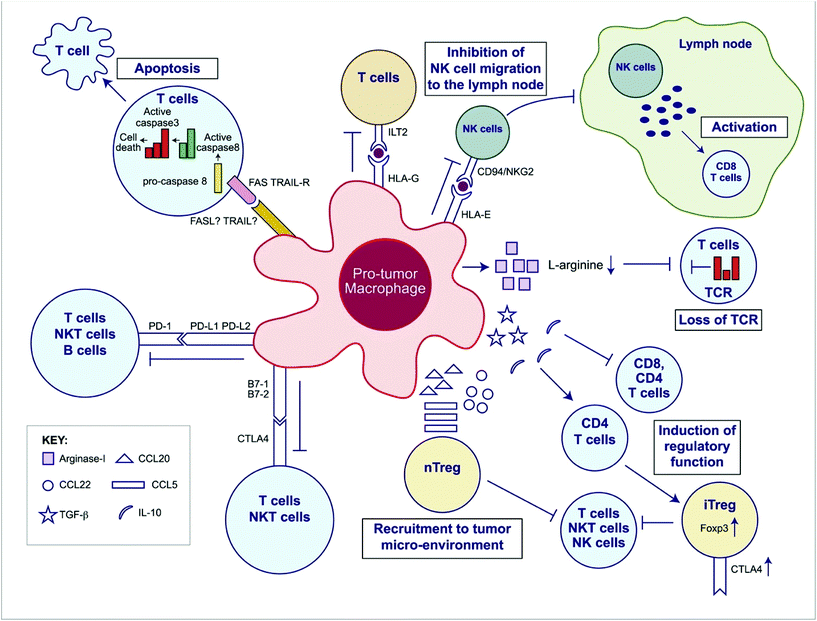 | ||
| Fig. 2 Overview of the immunosuppressive mechanisms of tumor-associated macrophages (TAMs). TAMs express an array of effector molecules that inhibit antitumor immune responses; this includes cell surface receptors, cytokines, chemokines, and enzymes. Inhibition of immune responses by direct cell-to cell-contact is based on the interaction of TAM receptor ligands with their counterpart death and/or inhibitory receptors expressed by the target effector cells. TAMs express the ligand receptors for PD-1 and CTLA-4 that upon activation suppress cytotoxic functions of T cell, natural killer (NK) T cells, and NK cells. TAMs also express the ligand for the death receptors FAS and TRAIL that triggers caspase-dependent cell death (apoptosis) in target cells. TAMs also express the nonclassical HLA-G that inhibits T cell function through interaction with the costimulatory signal of T cells ILT2 and HLA-E that inhibit NK cells through CD94 (also known as NKG2). TAMs secrete the cytokines IL-10 and TGF-β that inhibit T cell effector functions and induce regulatory functions and chemokines CCL5, CCL20, and CCL22 that recruit nTreg cells. TAMs secrete Arginase I that inhibit TCR ζ chain re-expression inactivated T cells by the depletion of L-arginine. (Adapted from Noy and Pollard (2014).36 Copyright 2014 Elsevier.) | ||
To properly understand therapeutic approaches that target TAMs, it is important to know how the phenotype of macrophages impacts their function. According to the macrophages balance hypothesis, macrophages phenotype can be classified into two main categories: the classically-activated M1 phenotype and the alternatively-activated M2 phenotype.41 The phenotype exhibited by a macrophage is determined by environmental cues that dictate the type of functions it performs. The M1 phenotype is induced by Th1 cytokines (e.g. interferon-gamma, (IFN-γ)), toll-like receptor (TLR) agonism, and bacterial moieties (lipopolysaccharide). Macrophages expressing this phenotype perform pro-inflammatory activities such as microbe clearance and tumor eradication, antigen presentation, and IL-12 secretion. On the other hand, the M2 phenotype is induced by Th2 cytokines (IL-4, IL-13) and leads to anti-inflammatory functions such as wound healing, angiogenesis, and the secretion of IL-10.41–43 TAMs are generally skewed toward the immunosuppressive M2 phenotype and support tumor progression (i.e. are pro-tumor).
Recently, we and others discovered that the role of immunosuppressive myeloid cells can be expanded further in the aftermath of cytotoxic cancer therapy.44–46 In this setting, wide scale apoptosis of cancer cells in response to cytotoxic drugs initiates a process known as efferocytosis – or apoptotic cell clearance by neighboring phagocytes.44 Efferocytosis prevents secondary necrosis of apoptotic cell debris, limiting pro-inflammatory damage associated molecular pattern (DAMP) release. At the same time, efferocytic macrophages polarize to an M2-like phenotype, secrete anti-inflammatory cytokines (IL-10, IL-13, and TGF-β), and recruit FoxP3+ Treg cells.44,47 Blockade of efferocytosis after cytotoxic therapy can reverse immunosuppression and significantly reduce the growth and metastasis of tumor residual disease.
Pattern recognition receptors (PRRs) recognize and alert the immune system when an organism is invaded by harmful, foreign pathogens. They recognize pathogen-associated molecular patterns (PAMPs) on pathogens and activate pathways that stimulate an immune response.51 Common PRRs include, among others, the stimulator of interferon genes (STING) pathway, retinoic acid-inducible gene I- (RIG-I)-like receptors (RLRs), and Toll-like receptors (TLRs). PRRs like STING, RLRs, and TLRs are present in many cell types, including cancer cells, macrophages, DCs, and others,52–56 where their intracellular location varies. STING and RLRs reside in the cytosol, whereas nucleic acid sensing TLRs are located primarily within endosomal vesicles.
The STING pathway is activated by cyclic dinucleotides (CDN) such as cyclic-di-AMP. This activation initiates a cascade resulting in the production of pro-inflammatory type 1 IFNs, inducing CD8α+ DCs to cross-present antigen to T cells and prime T cell-mediated antitumor immunity.57–59 STING plays an important role in mediating adaptive immune responses. Thus, STING-deficient mice show greater susceptibility to tumor formation and have impaired antitumor immunity.57 Moreover, STING is important for immune checkpoint blockade-mediated T cell responses as immune checkpoint administration in STING-deficient mice has abrogated T cell responses.56 Thus, the expression of STING in many cancer cells makes it a good therapeutic target for treating non-immunogenic tumors, particularly those resistant to checkpoint inhibitors.58
On the other hand, RLRs recognize viral RNA or viral replication intermediates and initiate an innate immune response.51,60 The RLR family comprises of RIG-I, melanoma differentiation association protein 5 (MDA-5), and laboratory of genetics and physiology 2 (LGP2).60 RIG-I is activated by double stranded RNA sequences containing 5′-diphosphate (5′-ppRNA) or triphosphates (5′-ppp RNA)52–54,61–63 while MDA5 is activated by polyinosine-polycytidylic acid (poly(I:C)).51 Recognition of these viral RNAs by RIG-I and MDA5 results in the activation of nuclear factor kappa-light-chain enhancer of B cells (NF-κB) and interferon regulator factor 3 (IRF3) to produce type I and III interferons and other pro-inflammatory cytokines.
TLRs are a broad family of Drosophila toll homologues that are located either on the cell membrane or in the endosome of the cell. They contain an extracellular leucine-rich-repeat (LRR), a transmembrane domain and an intracellular domain called TIR (Toll/IL-1 receptor).64,65 TLRs can recognize a wide variety of PAMPs from lipopolysaccharides (LPS) on the bacterial cell wall to viral RNA.64 Recognition of nucleic acid-based agonists is however restricted to the endosomal TLRs (TLR 3, 7/8, and 9). Specifically, TLR 3, 7/8, and 9 are activated by dsRNAs, ssRNAs, and CpG-ODN, respectively.64 Just like RLRs, the activation of TLRs leads to NF-κB-mediated release of pro-inflammatory cytokines66,67 and APC maturation.68
2. Classes of immunostimulatory biomaterials
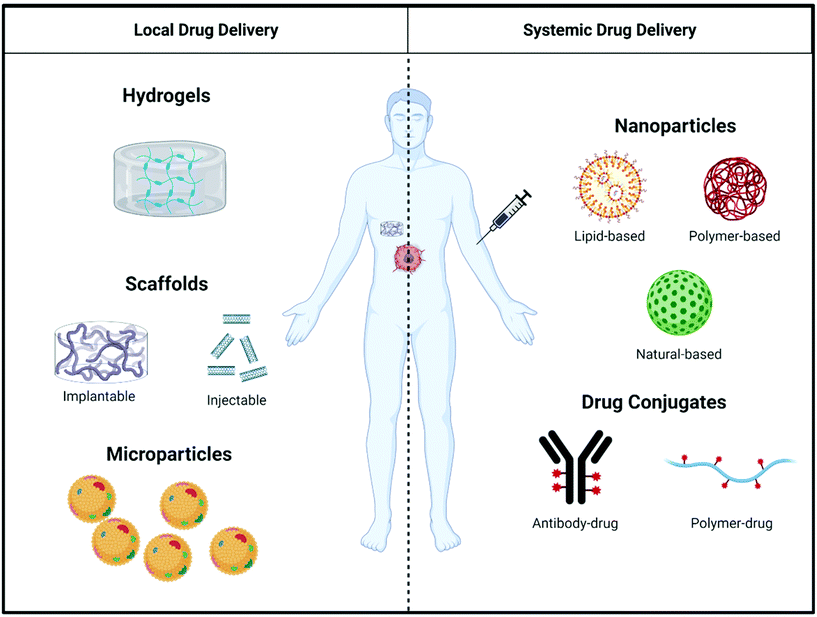
Even though immunostimulatory treatments have proven effective to counteract immunosuppression in the tumor microenvironment, shortcomings such as a lack of response in extremely ‘cold’ tumors69 and the toxicity of systemically-administered immunostimulatory drugs limit the effectiveness of existing therapies.70,71 To mitigate these shortcomings of immunostimulatory drugs, biomaterials can be used as a delivery vehicle to alter pharmacokinetics and biodistribution, and control release of therapeutic agents targeting the immune system. This section outlines the general design considerations of biomaterials used for both local and systemic delivery (Fig. 3) of immunostimulatory therapies to treat cancer.2.1 Biomaterials for the local delivery of immunostimulatory therapies
In order to combat systemic toxicity and off-target side effects often encountered during the systemic delivery of immunostimulatory drugs, researchers are investigating a more localized approach to immunostimulation leveraging macroscale drug delivery devices and biomaterials. Taking a local approach in immunostimulation of the tumor microenvironment allows for a focused administration of the cancer treatment that directly affects the tumor and immune cells infiltrating the tumor, offering several advantages over systemic immunostimulation.72–75 Local immunostimulatory biomaterials deliver low doses of immunostimulatory agents proximal to the treatment site, circumventing issues of systemic toxicity. Additionally, local drug delivery by biomaterials can be optimized for spatiotemporal control of drug release to optimize the immune response using a variety of properties such as the rate of polymer degradation, diffusion mechanism, and affinity between the biomaterial and drug to tune drug release profile.74Hydrogels can be created using natural polymers, synthetic polymers, or a combination of the two.75,79–82 Natural polymers have intrinsic bioreactive and biocompatible properties that closely mimic the extracellular matrix (ECM). These polymers often have a high degree of biodegradability and degrade into natural byproducts that are easily cleared by the body. Synthetic polymers offer the ability to tune the hydrogel properties based on the chemical functionality of the polymer, are often nonimmunogenic, and do not interact with the cellular environment. To encourage biological interactions, synthetic polymers can be conjugated to biological ligands and proteins recognizable by host cells. While some synthetic polymers pose a risk of toxicity, many synthetic polymers used in hydrogel development have been tested widely and are FDA-approved. To overcome limitations of hydrogels composed of purely natural or synthetic polymers, hybrid polymers are being studied to combine the best features of both material classes. The customizability of hydrogels allows for hydrogels to be designed to respond to various environmental stimuli, including pH, temperature, oxidative stress, and enzymatic activity.82 Stimuli-responsive hydrogels offer “smart” systems capable of responding to environmental cues and tightly regulating material response based on biological processes occurring within the microenvironment of the hydrogel.
Like hydrogels, modifying design parameters of scaffolds can change the physical properties of the matrix, impact diffusivity of immunostimulatory factors to surrounding tissue, and bioreactivity. Diffusion is an important factor for scaffolds in aiding immune cell recruitment and survival as well as controlled drug release. The diffusion coefficient, as well as the drug loading capability, of the scaffold is dependent on the porosity of the matrix. Pores in scaffolds can be introduced by sparging air or carbon dioxide as the 3D matrix sets, through the generation of a gas by the cross-linking process,87 particulate or salt leaching,87 or the use of 3D printing to create scaffolds with controlled degrees of porosity to tune the drug release profile.88 Additionally, the efficacy of scaffolds can be enhanced through surface modification. Some synthetic polymers have poor bioreactivity which can hinder cell recruitment and activation. However, this can be altered through surface modification such as in the MSR scaffolds modified with poly(ethylene glycol) (PEG), PEG-RGD (PEG-integrin-binding ligand Arg-Gly-Asp), and PEG-RDG (Arg-Asp-Gly) groups by Li et al.89
2.2 Biomaterials for the systemic delivery of immunostimulatory therapies
Systemic administration of immunostimulatory drugs is a promising approach for the treatment of metastatic cancers that have spread to distant sites throughout the body as well as the treatment of primary tumors. Even though many immunotherapies have gained FDA approval,71,92–94 systemic administration of the drugs have multiple drawbacks.93,94 The dose of immunostimulatory drugs given systemically is limited by concerns over toxicity.95 Large portions of the systemically administered drug fails to reach the target site, instead biodistributing to other organs, limiting on-target efficacy and increasing off-target toxicity.82,96 To address the poor natural pharmacokinetics and biodistribution of many immunotherapeutic drugs, numerous immunostimulatory biomaterials have been developed for systemic delivery. Here, we focus our attention on two major classes of systemic delivery materials, nanoparticles and drug conjugates.While therapeutic nanoparticles can be fabricated using a variety of methods, there are key design parameters that must be considered in order to create an effective delivery system. The size of nanoparticles plays a key role in nanoparticle accumulation at tumor sites and clearance by phagocytes. Though the enhanced permeability and retention (EPR) effect is variable and mechanisms to better understand nanoparticle accumulation in tumors are being elucidated, tumors that do contain leaky vasculature and impaired lymphatic drainage allow circulating nanoparticles to preferentially infiltrate the tumor and avoid clearance from the tumor interstitium. Moreover, it is now appreciated that nanoparticles can enter tumors by a variety of mechanisms, including passively via EPR,99,100 dynamically via vascular vents,101 actively via endothelial transport,102 and via hitchhiking on-board phagocytes.103 One study of note was conducted by Perrault et al. to systematically study how particle size (10–100 nm) influenced the pharmacokinetics of nanoparticles.104 The authors showed that 60–100 nm diameter particles had the highest tumor accumulation but smaller particles like the 20 nm diameter nanoparticles tested have improved tumor penetration. Researchers must determine the acceptable size range for nanoparticles to optimize accumulation and permeation within their particular application – though for most cases, nanoparticles in smaller size ranges (∼20–50 nm) appear most suitable in order to balance tumor uptake and penetration within the tissue.
Other key design considerations include the material of the particle, surface charge, and degradation mechanism.105,106 Nanoparticles with a positively charged surface have a higher rate of cellular uptake while neutral and slightly negatively charged surfaces reduce cellular uptake. Due to the increased cellular uptake of positively charged nanoparticles, in addition to rapid protein adsorption and aggregation, these particles generally have very short circulation times. For this reason, charged materials are often coated with materials like PEG to shield surface charge, increase biocompatibility, and stealth the particles from the mononuclear phagocyte system (MPS). The degradation mechanism and drug release trigger can be modified to suit a wide range of applications. Some of these triggers include tumor hypoxia, low pH of endosomes and tumor microenvironment, tumor-specific enzymes, and oxidative stress. These triggers cause biodegradation of the nanoparticle material while also enabling a mechanism for drug release from the particles. For further analysis, see the extensive review on linker chemistry design and nanoparticle drug release by Wong and Choi.106
Importantly, recent studies revealing the role of immune cells in nanoparticle transport and antitumor efficacy generate excitement about the potential of nanomedicines specifically in the area of cancer immunotherapy. A series of elegant studies from the Weissleder group indicate that large portions of nanoparticles reaching tumors enter TAMs rather than tumor cells.107–111 Marios Sofias et al. showed that αvβ3-targeted particles accumulate in tumors via phagocyte hitchhiking rather than by canonical receptor–ligand binding within the tumor.103 In this study, both targeted and non-targeted nanoparticles were engulfed by circulating phagocytes (e.g. neutrophils and monocytes) and shuttled to tumors. Korangath et al. recently studied the impact of antibody-functionalization of nanoparticles on tumor accumulation in models of breast cancer.112 Rather than finding that Trastuzumab- (anti-HER2 antibody) functionalized nanoparticles colocalized with HER2+ tumor cells, they found the particles largely colocalized with immune cells in tumors. Further, differences in tumor accumulation of Trastuzumab-functionalized and control IgG-functionalized nanoparticles were not significant, even in HER2+ models of breast cancer. Rather, tumor accumulation changed significantly between immune-competent and immune-compromised models, suggesting that nanoparticle biodistribution was not dictated primarily by active targeting to the HER2 receptor but by immune status. These studies, and others, indicate that nanoparticles target immune cells efficiently in vivo, motivating further studies to probe immunobiology–nanomaterial interactions and signifying the potential promise of nanotechnology to improve cancer immunotherapy.
In cancer therapy, monoclonal antibody-based drugs recognize specific antigens on or near the tumor site to elicit a cytotoxic response, but therapeutic effects can be augmented through conjugation.113 Antibody–drug conjugates utilize the targeting capabilities of monoclonal antibodies and the cytotoxic/immunotherapeutic effects of the conjugated drug.113,114 The basic design of these conjugates consists of the antibody, a linker, and the drug. Any of these three components can be modified to engineer the system toward the application of choice.115 Once an antibody is chosen that will provide specific binding, other properties must be considered such as antibody stability after conjugation, in systemic transit, and at the site of tumors or immune organs. The linker plays an important role in the stability and drug release of antibody–drug conjugates. These linker components can be sensitive to lysosomal enzymes, pH-responsive, or responsive to glutathione (an intracellular reducing agent). Some antibody–drug conjugates utilized non-cleavable linkers. In these cases, the payload can only be released once the conjugate is taken into the cell and the antibody is degraded. After determining the best antibody and linker to suit the application, the site of conjugation onto the antibody is another important consideration as the conjugation site greatly impacts the activity of the drug conjugate. Most researchers use alkylation of reduced interchain disulfides, acylation of lysine residues, or alkylation of genetically engineered cysteine residues to conjugate the drug and linker to the antibody.116
Polymer–drug conjugation allows researchers to modify the pharmacokinetics of immunotherapy drugs, protect the drug from harsh in vivo environments, and incorporate targeting moieties, all within a single molecule.117 Conjugating immunotherapeutic or cytotoxic drugs to a synthetic polymer such as PEG protects the drugs from enzymatic degradation and rapid clearance via the liver and kidneys.118,119 As a result, polymer–drug conjugates generally increase circulation time compared to the parent drug. Polymer–drug conjugates depend on passive accumulation at tumor sites and can be further modified with targeting ligands in order to bind specific immune cell targets or cancer cells.117 Conjugating small-molecule drugs to polymer chains offers several advantages such as improved solubility, increased drug stability, prolonged circulation half-life, and altered biodistribution.118 Today, many drug conjugates have been approved by the FDA or are being tested in clinical trials.114,117 Current advances in oncology research have primarily used antibody- and polymer–drug conjugation to deliver cytotoxic drugs; however, it is theoretically possible to replace the drug component with common immunotherapies in future iterations.115,117 By conjugating drugs to polymers and/or antibodies, researchers achieve a slower clearance rate, prolonged drug circulation, targeted, delivery and can alleviate toxic, dose-limiting side effects of systemic immunotherapy.
In summary, local and systemic biomaterials-based delivery systems overcome the limitations that attend the use of immunostimulatory drugs by favorably altering their pharmacokinetics and biodistribution to enhance efficacy in vivo. Local biomaterial delivery systems can be fixed close to the target site allowing for low doses of immunostimulatory drugs to achieve efficacious concentrations in the target area while minimizing systemic toxicity. Injectable hydrogels and scaffolds possess greater flexibility than implantable scaffolds and can be used to locally deliver immunostimulatory drugs in hard-to-reach or inoperable tumors which are inaccessible to implantable scaffolds (usually utilized at tumor resection sites120). They also avoid unnecessary tissue damage that accompanies surgical implantation and require less expertise to be administered.120 Moreover, they more uniformly interact with the local tissue microenvironment because they conform to natural cavities and other available spaces before forming a rigid structure. However, injectable scaffolds are limited by the type of materials available for use in their fabrication as few materials possess mechanical properties that allow needle injection followed by hardening in vivo.120
Systemic delivery systems can deliver immunostimulatory drugs to multiple target sites at sufficient doses throughout the body while minimizing toxic side effects. This makes them particularly advantageous when treating metastatic cancers which have disseminated beyond a single point of origin. Nanoparticles make use of both active and targeting mechanisms to preferentially deliver cargo to tumors. However, the physical and biological barriers encountered by nanoparticles in circulation significantly impacts the number of nanoparticles that reach their intended destination.72,121 A recent review by Wilhelm et al. analyzes probable phenomena that affect the efficiency of nanoparticle delivery and proffers strategies to overcome these limitations.121
3. Biomaterials that turn ‘cold’ tumors ‘hot’
Today, researchers are applying materials such as hydrogels, scaffolds, microparticles, nanoparticles, and drug conjugates to overcome a myriad of drug delivery challenges in immunoengineering. Lessons learned from the past few decades of interdisciplinary materials science, drug delivery, biomaterials, cancer biology, and immunology research are being leveraged for the rapid development and translation of immunostimulatory biomaterials that can improve clinical outcomes in cancer immunotherapy. Recent advances in the use of biomaterials to boost tumor immunogenicity and improve cancer immunotherapy are reviewed below and summarized in Table 1.| Immunotherapy | Approach | Material | Cargo | Ref. |
|---|---|---|---|---|
| Cytokine therapy | Hydrogels | mPEG-PLGA | GM-CSF | 122 |
| Dextran, L-lactide, D-lactide | IL-2 | 123 | ||
| Hyaluronic acid | IFN-α2a | 124 | ||
| PMNT-PEG-PMNT | IL-12 | 125 | ||
| PEGylated poly(L-valine) | Tumor cell lysate, Poly(I:C) | 126 | ||
| Scaffolds | Polyglyconate and porcine gelatin drug-eluting scaffold | CCL17 | 127 | |
| Antibody–cytokine conjugates | scFV diabody specific to EDA of fibronectin (F8) | IL-4 (F8-IL-4) and IL-12 | 128 | |
| F8 | IL-13 (F8-IL-13) and IL-12 | 129 | ||
| Lipid nanoparticles | Phosphatidylcholine | TRAIL-conjugated ssDNA; two types of ssDNA, DNA-FD and DNA-RD, encapsulated in separate liposomes | 130 | |
| Polymeric nanoparticles | PBAEs | IL-12 | 131 | |
| Pathogen recogniztion receptor agonist | Hydrogels | K2(SL)6K2 multidomain peptide | Cyclic dinucleotide STING agonist | 132 |
| Scaffolds | Hyaluronic acid | 2′3′-cGAMP (STING agonist) or R848 (TLR7/8 agonist) | 133 | |
| Alginate | STING agonist, CAR T cells | 134 | ||
| Lipid nanoparticles | Phosphatidylcholine, DOTAP | cGAMP (STING agonist) | 135 | |
| DOTAP, cholesterol, DSPE-PEG | cGAMP | 136 | ||
| POPE, DMG-PEG, YSK05 | STING agonist | 137 | ||
| Microparticles | PLGA | Poly(I:C) | 138 | |
| Polymeric nanoparticles | PBAEs | cGAMP | 139 | |
| PEG, DEAEMA, BMA, PDSMA (polymersome) | STING agonist | 57 | ||
| DMAEMA, BMA, PAA | STING agonist | 55 | ||
| Co-delivery of immune stimulating signals | Hydrogels | Hyaluronic acid | Ovalbumin expressing plasmid, GM-CSF | 140 |
| mPEG-b-poly(L-alanine) | GM-CSF, melanoma tumor cell lysates, anti-PD-1, anti-CTLA-4 | 141 | ||
| Microparticles | PLGA | Ovalbuimin and CpG-ODN | 142 | |
| PLGA-PEI | CpG-ODN, IL-10 siRNA, pDNA | 143 | ||
| Lipid nanoparticles | DSPE-PEG | IL-2, TGF-β inhibitor | 144 | |
| DOPC, cholesterol, DSPE-PEG, and DSPE-PEG-maleimide | Anti-CD137 and IL-2-Fc | 145 | ||
| Egg POPC, cholesterol, DSPE-PEG-maleimide, and iRGD coating | PI-3065 (P110δ inhibitor), 7DW8-5 (invariant natural killer T cell agonist, iNKT) | 146 | ||
| Infection mimicking | Scaffold | PLGA | GM-CSF, CpG-ODN, melanoma tumor lysates | 147 |
| PLGA | GM-CSF, CCL20, or Flt3L in combination with CpG-ODN and melanoma tumor lysates | 148 | ||
| Mesoporous silica rods | GM-CSF, CpG-ODN, ovalbumin | 85 | ||
| Macrophage reprogramming | Lipid nanoparticle | Phosphatidylcholine | CSF1R inhibitor, SHP1 inhibtor | 149 |
| Polymeric nanoparticle | PBAEs | mRNA | 150 | |
| β-Cyclodextrin | R848 | 107 | ||
| Modulating antigen trafficking | Polymeric nanoparticle | PAA, DMAEMA | Ovalbumin | 151 |
| PAA | Ovalbumin | 152 | ||
| Microparticle | PLGA | Ovalbumin | 153 | |
| Artificial antigen presenting cells | PLGA | Melanoma antigen | 154 | |
| PEG, PLGA, | H-2K TRP2 Ig dimers, anti-CD28, CD47-Fc | 155 | ||
| PLGA | Ovalbumin SIINFEKL peptide, anti-CD28, rhIL-2 | 156 |
3.1 Biomaterials that improve cytokine delivery to tumors
Cytokines play an essential immunostimulatory role in the tumor microenvironment. Some cytokines serve to activate T and NK cells that attack cancer cells144 while others aid tumor growth, survival, and metastasis.157–159 Cytokines work in an autocrine or paracrine fashion and short half-lives ensure their effects in normal physiology are usually localized.160 In therapy however, cytokines are often administered systemically and at high doses (to ensure sufficient doses reach the target site to achieve therapeutic effects).161 The systemic administration of high doses of soluble cytokines, however, leads to dose-dependent toxicities159 such as vascular leak syndrome,162,163 hypotension,164 and thrombocytopenia.164 The attempt to limit these toxic side effects can prevent the administration of curative doses.165 Several local and systemic drug delivery approaches can be leveraged to improve the biodistribution of cytokines to tumors in vivo.Implantable scaffolds have also been used to improve cytokine delivery. In one example, drug-eluting scaffolds grafted with pancreatic cancer tissue were implanted in mice for local delivery of CCL17.127 These scaffolds were made of polyglyconate and porcine gelatin. Their release profile showed sustained release of CCL17 for 7 days with a cumulative release of 72%. These scaffolds recruited 5.7-fold and 6.6-fold more CTLs to the cancer tissue than non-eluting scaffolds and the control group, respectively. Additionally, these CCL17-loaded scaffolds reduced the weight of the tumor by 1.6-fold and 1.4-fold compared to non-eluting scaffold and the control group, respectively. In sum, using local delivery systems to dispatch cytokines offers the advantage of boosting the local immune response against cancers at doses that are safe for in vivo administration.
3.1.2.1 Antibody–cytokine conjugates. Antibody–cytokine conjugates can be used to achieve targeted delivery of cytokines to tumors, reducing off-target accumulation and systemic toxicity.128,160,161,165 Here, the antibody has a strong affinity for targets highly abundant in the tumor microenvironment.160,166 These targets include antigens such as extra domain A (EDA) and B (EDB) of fibronectin which are strongly expressed in a majority solid tumors and lymphomas; cellular targets such as integrins (αvβ3), annexin A1, and others;166 and ligands whose expression is largely confined to the tumor microenvironment.160 Antibody–cytokines conjugates can be divided into three categories: (1) whole antibody–cytokines conjugates, (2) Fc fragment–cytokine conjugates, (3) and cytokines fused to antigen binding fragments such as scFv, Fab fragment, and others.167
Antibody–cytokine conjugates have been used for targeted delivery of cytokines such as IL-2, GM-CSF, IL-12, IL-4, TNF, and the chemokine CXCL-10 and represent a promising biomaterials-based approach to improve the pharmacokinetics and tumor delivery of cytokines in vivo.128,161,168,169 As an example, a study in F9 teratocarinoma murine tumor models, showed that antibody–cytokine conjugates facilitate higher cytokine accumulation in tumors compared to untargeted cytokines and completely eradicate tumors when co-administered.128 An antibody named F8, an scFV diabody fragment specific for EDA of fibronectin, was used to deliver IL-4 (F8-IL-4) in combination with IL-12. In the F9 tumor model, F8-IL4 had a higher accumulation in the tumor with a 6-fold increase in the percent injected dose per gram of F8-IL4 in the tumor compared to IL-4 conjugated to non-targeted antibody. Also, there was about 6-fold reduction in tumor volume of F8-IL-4 treated-mice compared to non-targeted IL-4.
3.1.2.2 Nanoparticles for cytokine delivery. Multiple nanoparticle approaches with controlled release mechanisms have been designed to improve cytokine delivery to the tumor microenvironment in therapeutic doses while simultaneously minimizing toxicities associated with systemic delivery. One approach by Wang et al. incorporated a pH-responsive monomer (2-(4-imidazolyl)ethylamine) into nanoparticles made of poly (β-amino esters) (PBAE) copolymers to deliver IL-12 to TAMs in B16F10 murine melanoma models.131 The nanoparticles were tailored to dissociate at the pH of the tumor microenvironment, releasing IL-12 in tumors to repolarize TAMs. Measurement of the concentration of IL-12 in the tumor microenvironment 48 h post injection showed a about 2.5-fold and over 6-fold increase in the concentration IL-12 compared to intratumoral and intravenous injection, respectively. Furthermore, there was a 2-fold increase in the number of TAMs with an M1 phenotype compared to free IL-12 as quantified by the expression of induced nitric oxide synthase (iNOS), a common marker of the M1 phenotype.
In another approach, nanoparticles were designed to release cytokines in response to stimuli in the tumor microenvironment.130 Here, the nanoparticles comprised of complementary DNA nanostructures encapsulated in a phosphatidylcholine liposome shell to deliver TNF-related apoptosis inducing ligand (TRAIL) to receptors on the cell membrane of human colorectal carcinoma cells. The DNA nanostructures where made through rolling circle amplification of ssDNA templates. Two types of DNA nanostructures were made; the first was made from ssDNA templates with an encoding sequence in the forward direction (DNA-FD) while the second encoding sequence was in the reverse direction (DNA-RD). The phosphatidylcholine liposomes contained an sn-2 acyl ester bond which is cleaved by phospholipase A2, an enzyme overexpressed in the microenvironment of various tumors. Analysis of the ability to deliver TRAIL to the cell membrane showed co-administration of nanoparticles containing DNA-FD and DNA-RD increased the percentage of membrane bound TRAIL from 42% to 76%, confirming that hybridization of both DNA nanostructures facilitates the interaction of TRAIL with the cell membrane receptors. In vitro cytotoxicity analysis showed that co-administration of nanoparticles containing DNA-FD and DNA-RD had an apoptosis ratio of 43.7% after 12 h compared to 31.7% and 29.6% when nanoparticles containing DNA-FD or DNA-RD where administered alone.
3.2 Biomaterials for improving delivery of nucleic acid-based PRR agonists
The use of nucleic acid-based agonists to induce signaling pathways stimulating innate and adaptive immunity has emerged as a promising therapeutic approach for boosting tumor immunogenicity. However, the administration of soluble agonists is attended with several difficulties. Many nucleic acid-based PRR agonists have low bioavailability because of their negative charge, small size, and highly hydrophilic nature.170 CDNs also suffer from rapid clearance before they reach the tumor and do not preferentially accumulate in tumors which can lead to off-target inflammation and autoimmunity.57,136 The lack of proper therapeutic responses following the soluble administration of these agonists motivates novel strategies to improve delivery and achieve safe, efficacious therapeutic responses.57,136 Several local and systemic delivery strategies have been used to improve delivery of these agonists.Scaffolds have also exhibited promising efficacy when loaded with PRR agonists. For example, Park et al.133 fabricated hyaluronic acid-based scaffolds to deliver either 2′3′-cGAMP or R848 (TLR7/8 agonist) to mice with resected 4T1 breast tumors. The use of R848-loaded scaffolds to extend the delivery of R848 enabled over 1.5-fold increase in the survival rate of mice compared to R848 and empty scaffolds after 90 days. A survival rate over 1.5-fold higher was also seen in mice treated with 2′3′-cGAMP-loaded scaffolds compared to mice treated when 2′3′-cGAMP and empty scaffolds after 90 days. This study showed that the controlled and localized release of R848 and 2′3′-cGAMP was crucial to survival. Other biomaterials used as implantable scaffolds with promising outcomes include alginate134 and mesoporous silica rods.85 In summary, macroscale delivery strategies achieve sustained and localized release of PRR agonists and have the potential to significantly enhance therapeutic efficacy in local settings.134
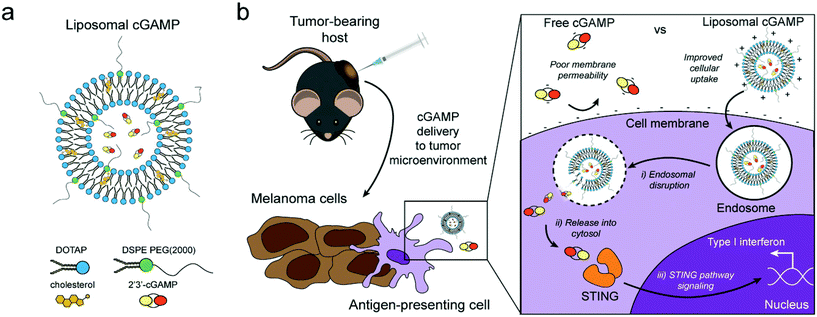 | ||
| Fig. 4 Schematic of liposomal cGAMP structure and therapeutic strategy. (a) 2′3′-cGAMP is encapsulated in cationic liposomes created from 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and cholesterol using thin film rehydration, freeze thawing, and membrane extrusion. A polyethylene glycol(PEG)-containing lipid (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]; DSPE-PEG(2000)) is optionally included in the liposome preparation to create a PEG coating that improves liposome stability. (b) In a therapeutic setting, melanoma tumor-bearing hosts are injected with free or liposomal cGAMP, where cells, for example antigen-presenting cells (APCs), in the tumor microenvironment take up liposomal cGAMP concurrent with melanoma cell antigens. (Inset) Free cGAMP has limited transport into the cytosol due to the presence of two negative charges that limit its permeability through the negatively charged cell membrane. cGAMP encapsulated in cationic liposomes shows improved cell membrane binding and uptake. Once internalized into the endosomal compartment, cationic liposomes facilitate the release of cGAMP into the cytosol, where cGAMP binds to the stimulator of interferon genes (STING) adaptor molecule, leading to type I interferon production by the APC (adapted from Koshy et al. (2017).136 Copyright 2017 John Wiley & Sons, Inc.). | ||
Biodegradable polymer-based nanoparticle delivery platforms that contain cationic materials have also been used to enable the delivery of negatively charged nucleic acid-based PRR agonists. PBAEs are one class of cationic polymers used in these type of nanoparticles and has been used to deliver STING agonists.139 In this study by Wilson et al., cCAMP-loaded nanoparticles were co-administered with anti-PD-1 to boost immunity against B16F10 murine melanoma tumor models. After 18 days, there was a 3-fold decrease in tumor volume compared to soluble cGAMP combined with anti-PD-1 immunotherapy.
In addition, delivery of PRR agonists mediated by polymersomes has shown increased CTL infiltration and enhanced anti-tumor activity of checkpoint inhibitors.57,58 For example, polymersomes designed by Shae et al.57 were composed of a cationic block which imparts electrostatic complexation and cargo protection along with an endosome-destabilizing terpolymer block that mediates efficient cytosolic delivery. These nanoparticles were used to deliver cGAMP to B16F10 murine melanoma tumor models. The intratumoral administration of cGAMP-loaded nanoparticles showed an 11-fold decrease in tumor growth compared to soluble cGAMP. Also, delivery mediated by cGAMP-loaded nanoparticles resulted in one-third of mice completely rejecting tumors compared to 100% tumor penetrance for mice given free cGAMP.
Furthermore, polymeric micelles featuring a similar design strategy were used to deliver RLR agonists to tumors to mediate immunogenic cell death.52,55 Jacobson et al.55 used endosomolytic polymeric micelles to deliver 5′-ppp-RNA to CT26 murine tumor models and measured the amount of Annexin V and 7-AAD double positive cells indicating cell apoptosis and necrosis, respectively. The results showed that the 5′-ppp-RNA loaded micelles increased the percent of cells double positive for Annexin V and 7-AAD 5-fold compared to empty micelles. In vivo results showed the combination of anti-PD-1 and the 5′-ppp-RNA-loaded micelles resulted in about 30% survival rate compared to no survival in the mice given PBS and anti-PD-1 therapy.
3.3 Biomaterials for the co-delivery of immune agonists
Adjuvants are regularly co-administered with subunit antigens to boost immunogenicity of the antigen. For this combination to be maximally effective, both antigen and adjuvant should be present within the same APC.171 However, the soluble administration of antigen and adjuvant cannot guarantee that they are presented to the same APC on similar timescales. To ensure antigen and adjuvant co-delivery, biomaterial-based dual delivery systems have been developed that can co-encapsulate and deliver multiple molecules simultaneously. Several studies have reported improved immune response when co-delivering antigen and adjuvant using biomaterial-based delivery systems.126,172–174 In one study, Wilson et al. investigated the use of pH-responsive polymeric micelles to co-deliver ovalbumin and CpG ODN, incorporating both within sub-100 nm particles.175In vitro studies showed a 5.5-fold and 2.5-fold increase in uptake of ovalbumin and CpG ODN when the micelles were used compared to soluble ovalbumin and CpG ODN. In vivo studies showed an 18-fold increase in the amount of IFN-γ-positive CTLs compared to the soluble administration of both antigen and adjuvant.In addition, dual delivery systems can be used to deliver signals that initiate multiple pathways that synergistically boost anti-tumor immunity.17,144 The rationale behind this concept is that tumors use multiple immunosuppressive avenues to escape eradication by the immune system. Often, this results in diminished efficacy of single immunotherapies. For instance, the secretion of immunosuppressive cytokines can downregulate NK and T cell activity when pro-inflammatory cytokine therapy is being administered, resulting in reduced therapeutic efficacy of the pro-inflammatory cytokine.144 Inhibiting the effects of immunosuppressive signals produced by tumor cells while concomitantly administering pro-inflammatory molecules can significantly boost anti-tumor immunity.144 Combination therapies can, however, be toxic if administered in soluble form. Therefore, delivery systems that can deliver multiple immune stimulating molecules while minimizing toxicity could significantly improve treatment outcomes.176
In a seminal study, Park et al. developed biodegradable core–shell nanogels to co-deliver IL-2 and a TGF-β inhibitor to aggressive melanomas.144 These nanoscale liposomal and polymeric gels were composed of phosphatidylcholine and the lipid–polymer conjugate 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG) forming an external lipid bilayer around a polymeric nanogel core (hydrophilic for encapsulation of IL-2). Methacrylate-conjugated-β-cyclodextrins (β-CD) were included to enable hydrophobic drug loading into inclusion complexes of the β-CD. The nanoparticles showed sustained delivery of IL-2 and the TGF-β inhibitor, SB505124, over 7 days. Co-delivery of IL-2 and SB505214 in the nanoparticles resulted in a 5-fold increase in survival rate compared to soluble administration of the combination therapy. Also, analysis of CTL infiltration into tumors showed approximately 3-fold increase in the number of activated CTLs in the tumors compared to the control. Finally, the nanoparticles helped to avoid the toxicities associated with high-dose administrations of IL-2 which tends to abate its therapeutic benefits.
Another example that epitomizes the improvements of combination therapies delivered by biomaterials is that of Zhang et al.145 Here, IL-2 Fc or anti-CD137 were conjugated to the surface of PEGylated liposomes and pharmacokinetics were measured and compared to that of the soluble forms of IL-2-Fc or anti-CD137. The authors discovered that combined delivery of both agonists (encapsulated within liposomes) to B16F10-Trp2KO tumor xenografts increased tumor accumulation markedly. Liposomal anti-CD137 showed a 5-fold increase in tumor accumulation compared to free anti-CD137 at 4 h and 24 h while Liposomal IL-2-Fc showed 50% greater accumulation at 4 h than free IL-2F-Fc. The use of PEGylated liposomes to co-deliver IL-2 and anti-CD137 mediated similar anti-tumor activity to soluble agents while reducing the systemic toxicities associated with free administration of the agonists.
3.4 Infection-mimicking biomaterials
The creation of vaccines has proven to be one of the most important breakthroughs in medicine and has enabled the successful prevention and treatment of various diseases.177 Vaccines usually incorporate antigens against an infectious disease and adjuvants which serve as “danger signals” to enhance innate immune response against an antigen.178 The goal of using vaccines is to replicate the immune response generated by pathogens without inducing the negative effects associated with infection.179 Current cancer vaccines are therefore unable to provide entirely protective responses due to a lack of strong, long-lasting humoral and cellular immune responses that resemble those generated by pathogens.177,179 Novel biomaterial-based approaches that can mimic pathogen behavior to bring about prolonged immune activation and continued APC stimulation could be a solution to bring about more robust treatment responses.147,177,179Polymeric scaffolds can be used to create an infection-mimicking microenvironment in which exogenous cytokines, PRR agonists, and a cancer antigen are combined to enable precise control over the magnitude and kinetics of DC activation (Fig. 5).147,148 Cytokines are used to recruit DCs to the site of the scaffold where they are loaded with antigen and activated by adjuvants, both of which are immobilized within the scaffold.147 The polymeric scaffolds act as an antigen/adjuvant depot and delivery system to enhance recruitment and activation of DCs. They would mediate spatiotemporal control over delivery of immune stimulants to control cell activation. This approach mimics bacterial infections where cells are recruited by pro-inflammatory cytokines and are activated at the site of infection by PRR agonists. This approach was used by Ali et al.147 where they synthesized PLGA scaffolds to deliver granulocyte macrophage-colony stimulating factor (GM-CSF), CpG-ODN, and melanoma tumor lysates for improved priming of DCs. At high concentrations, GM-CSF gradients can entrap DCs at the site of the scaffold and preclude homing to the lymph nodes. However, the combination of CpG-ODN with GM-CSF enables DCs to leave the site of the scaffold and traffic to the lymph node. The authors showed the importance of using PLGA scaffolds as a residence for the DCs during activation by comparing to bolus injections of melanoma tumor lysates with CpG-ODN and GM-CSF. In a model of melanoma, the administration of tumor lysate, CpG ODN, and GM-CSF within PLGA scaffolds resulted in about 50% survival after 90 days compared to 0% (at day 40) for the bolus injections of the three molecules. After increasing the CpG-ODN concentration 10 times within the scaffolds, 90% survival was achieved after 90 days.147 Since this seminal work, multiple infection-mimicking systems have been developed to recruit and activate DCs.85,141
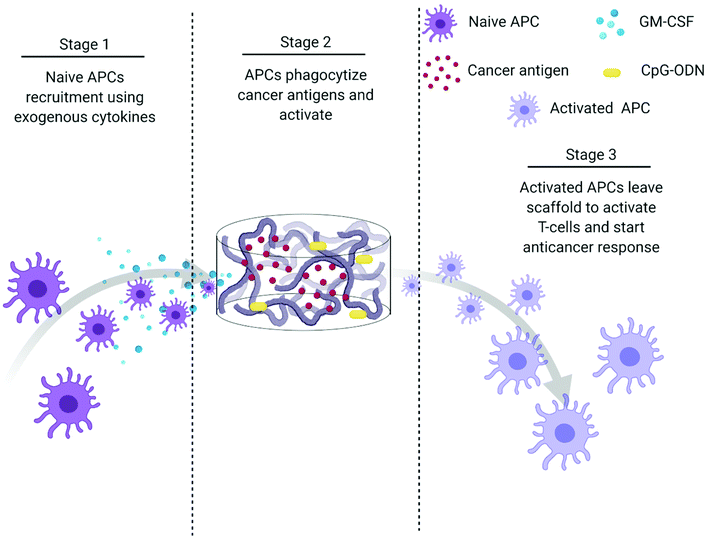 | ||
| Fig. 5 Process schematic of Ali et al. infection-mimicking scaffold design. Stage 1: Recruit naïve APCs using released GM-CSF. Stage 2: The recruited APCs reside in the matrix of the scaffold to be programmed using preloaded cancer antigens and adjuvants. Stage 3: The newly programmed APCs leave the scaffold to activate T-cells and initiate an anticancer immune response.147 | ||
Additionally, particulate delivery systems can be designed to mimic infection and boost the efficacy of antigens.177,180 Pathogen-mimicking nanoparticles are divided into three broad categories: synthetic particulate systems, virus-like particles, and bacterial outer membrane vesicles.181 Pathogen-mimicking delivery systems have been shown to enhance antigen delivery compared to nanoparticles made from the canonical polymeric materials. For example, inulin-acetate, a bioactive polymer, which activates TLR4 on DCs increased antigen concentration in DCs by 6.2-fold compared to PLGA nanoparticles with similar physical properties in vitro.177 Moreover, these inulin-acetate delivery systems show enhanced ability to induce humoral response.177 The ovalbumin-loaded inulin-acetate particles increased the total amount of ovalbumin-specific IgGs almost 30 times compared to ovalbumin delivered with the adjuvant alum. The potent adaptive immune responses generated by infection-mimicking systems indicate great potential for improving the response to cancer immunotherapy and warrant continued attention.
3.5 Macrophage reprogramming
TAMs are some of the most prevalent cells within many tumors and are known to support the growth and metastasis of advanced malignancies.36 TAMs can express a range of phenotypes, but most TAMs tend toward an M2, wound healing phenotype. The M2 macrophage phenotype mediates immunosuppressive activities in the tumor microenvironment and can provide tumors with resistance to immunotherapy.40,182 In contrast, the M1 phenotype is anti-tumoral and mediates important pro-inflammatory events that boost tumor immunogenicity.150 The plasticity of TAMs can be harnessed to program their phenotype toward anti-tumoral activities that enhance the response to immunotherapy.149Targeting pathways responsible for differentiating TAMs is a viable strategy to repolarize TAMs from an M2 phenotype to M1.149 Colony stimulating factor 1 (CSF1) is one important pathway linked to the recruitment and differentiation of TAMs to an M2 phenotype, and inhibition of this pathway has been shown to correlate with the downregulation of M2-associated genes and the upregulation of M1-associated genes.183 Accordingly, Ramesh et al. sought to reprogram TAMs to exhibit an M1 phenotype by delivering a CSF1R inhibitor in lipid nanoaprticles.149 They designed self-assembling lipid nanoparticles comprised of phosphatidylcholine and DSPE-PEG to deliver a CSF1R inhibitor and Src homology region 2 (SH2) domain phosphatase 2 (SHP2) inhibitor. The SHP2 inhibitor promotes phagocytosis by M1 TAMs and its combination with the CSF1R inhibitor enhances the anti-tumoral activity of M1 TAMs. In a 4T1 murine breast tumor model, the CSF1R-SHP2-nanoparticles decreased the percentage of M2 macrophages 5-fold and increased the percentage of M1 macrophages 3-fold compared to control. Also, CSF1R-SHP2-nanoparticles reduced tumor volume by about 3-fold compared to control. Another suitable target is the interferon regulatory factor 5 (IRF5) pathway. Zhang et al. designed hydrolytically-degradable nanoparticles comprised of cationic PBAEs to deliver in vitro-transcribed mRNA encoding IRF 5 and IKKβ to mice bearing ID8 ovarian tumors.150 The IRF5-IKKβ-nanoparticles resulted in a 17-fold decrease in the percentage of M2 macrophages and 20-fold increase in the percentage of M1 macrophages compared to control. Also, IRF5-IKKβ-nanoparticles increased median survival by over 2-fold compared to control and resulted in a 40% survival rate.
Rodell et al.107 synthesized nanoparticles made of β-cyclodextrin (β-CD) to deliver R848 to TAMs for repolarization in MC38 murine colorectal tumor models. Here, the use of nanoparticles with negative zeta potentials prevented undesirable uptake by hepatic cells. The cyclodextrin nanoparticles were made through amide bond formation between succinyl-β-CD and L-lysine. Though β-CD are not normally anionic, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 L-lysine to succinyl group molar ratio resulted in a negative zeta potential of −9.87 ± 0.59 mV. β-CD was used because the chemical structure mimics dextran molecules and has high avidity for macrophages. Repolarization of TAMs was measured in p40-IRES-eYFP-IL-12 reporter mice which co-express YFP with the M1 prototypical marker IL-12. Results showed approximately 2-fold increase in the average quantified expression of IL-12 compared to mice receiving soluble R848, reducing tumor growth 2–3-fold compared to vehicle control. Several other strategies such as microRNA delivery have also been used to reprogram TAMs with promising early results.184,185
2 L-lysine to succinyl group molar ratio resulted in a negative zeta potential of −9.87 ± 0.59 mV. β-CD was used because the chemical structure mimics dextran molecules and has high avidity for macrophages. Repolarization of TAMs was measured in p40-IRES-eYFP-IL-12 reporter mice which co-express YFP with the M1 prototypical marker IL-12. Results showed approximately 2-fold increase in the average quantified expression of IL-12 compared to mice receiving soluble R848, reducing tumor growth 2–3-fold compared to vehicle control. Several other strategies such as microRNA delivery have also been used to reprogram TAMs with promising early results.184,185
3.6 Modulating antigen trafficking
Antigen presentation is a vital process for generating successful tumor-specific immune responses. Tumors generate neoantigens which when recognized by APCs are taken up and processed for presentation to T cells on MHCs. CTLs are particularly effective at mediating robust anti-tumor immunity, but they require that antigen be presented on MHC-I. For presentation on MHC-I, antigens must either be cross-presented or delivered to the cytosolic MHC-I antigen processing pathway. However, the administration of soluble antigen usually leads to destruction in early endosomes before reaching the cytosol.152 Soluble antigens are also preferentially presented on MHC-II which primes T helper cells but not CTLs.186 These challenges limit the efficacy of soluble antigens to generate suitable CTL responses.Several particulate delivery systems have shown the ability to overcome these delivery challenges and augment antigen presentation on MHC-I.151,152 pH-Responsive polymeric nanoparticles are particularly suitable because they drive rapid cellular uptake of antigens and their endosomolytic features enable endosomal escape, providing the antigen access to MHC-I processing machinery in the cytosol.152 To our knowledge, the first work detailing the use of pH-responsive polymeric nanoparticles for protein antigen delivery in vivo was done by Foster et al.151 Here, poly(propylacrylic acid) (pPAA) was conjugated to ovalbumin and used either by itself or in combination with the cationic polymer pDMAEMA. The administration of pPAA–ovalbumin conjugates (with or without pDMAEMA) significantly reduced exocytosis of ovalbumin with 50% exocytosed in 4 h compared to the control which had 75% exocytosed in 30 min. Additionally, pPAA/pDMAEMA–ovalbumin conjugates had an 8-fold increase in ovalbumin-specific CTLs. This work was furthered by forming nanoplexes comprising of a MHC-I-restricted epitope from ovalbumin (SIINFEKL) that incorporated a cationic N-terminal decalysine tail and a three amino acid spacer to aid intracellular peptidase cleavage.152 The nanoplexes provided prolonged antigen presentation at higher magnitudes compared to free antigen. The use of nanoplexes to deliver antigen resulted in robust generation of antigen-specific CTLs whereas antigen alone generated CTL levels that were barely detectable. When combined with an adjuvant, the nanoplexes achieved a 10-fold increase in CTLs compared to the nonadjuvanted nanoplexes. This correlated with a prolonged duration of survival in melanoma tumor models (45 days for 100 percent death) compared to nonadjuvanted nanoplexes and free antigen with adjuvant (approximately 27 and 37 days, respectively). Co-loading of adjuvant inside the nanoplexes could possibly increase the number of CTLs cells even further.152
PLGA microparticles are another particulate delivery system that can modulate antigen trafficking for enhanced antigen presentation. Shen et al.153 studied the efficacy of PLGA microparticles for ovalbumin delivery with regards to antigen cross-presentation and CTL activation. Human DC-like cells co-cultured with ovalbumin-loaded PLGA microparticles generated comparable antigen cross-presentation to soluble ovalbumin at 1000-fold lower concentration. Measurements of the delivery of antigen to the cytosol showed that ovalbumin-loaded PLGA microparticles had a 36-fold increase in cytosolic antigen compared to ovalbumin delivered in soluble form. Though the authors could not highlight the mechanism by which microparticles enabled cytosolic delivery, Koerner et al.187 described two possible ways in which PLGA microparticles escape the endosome. The first involves the slow hydrolysis of the particle which leads to the gradual endosomal acidification making particles more positively charged and encouraging interaction with the endolysosomal membrane. The second mechanism is via the proton-sponge effect where the influx of hydronium and chloride ions during endosomal acidification increases osmotic pressure, leading to lysis of the endosomal membrane and antigen delivery to the cytosol. Other microparticles have been developed for enhanced antigen trafficking to achieve MHC-I antigen presentation and many of them incorporate adjuvants for CTL activation.142,187,188
Artificial antigen presenting cells (aAPCs) serve as another suitable strategy to improve antigen presentation as they can directly present antigen to CTLs, bypassing the challenges of cytosolic delivery.154,155,189 aAPCs are divided into two broad categories: cellular aAPCs which are made by genetically modifying living cells to present antigen and acellular aAPCs which are made from synthetic materials and modified with surface antigen and costimulatory signals to activate antigen-specific CTLs.156 Activation of T cells can be done ex vivo or in vivo. For ex vivo activation, studies showed that microsized-aAPCs are more effective at stimulating T cell response than nanosized aAPCs.156,190 However, for in vivo applications, nanosized-aAPCs tend to be more suitable because their small size enables better biodistribution.155 The shape of aAPCs play an important role in generating adequate T cell responses as well. Generally, ellipsoidal aAPCs generate higher CTL cell expansion than spherical aAPCs.154,155 This is because the aspect ratio of ellipsoidal aAPCs more closely mimics the immunological synapse of natural APCs.154 Furthermore, stealth can be added to aAPCs by PEGylation and the surface conjugation of CD47 which interacts with the signal receptor protein-α of MPS cells to inhibit phagocytosis.155 The use of these modified aAPCs resulted in 17.5-fold and 15.8-fold increases of antigen-specific CTLs in the blood and spleen, respectively, compared to control. Furthermore, CTL infiltration into tumors increased by 25.2-fold compared to control. These studies show that biomaterial-based platforms can modulate the trafficking and presentation of antigen to significantly boost cell-mediated responses against cancer.
Innovations in biomaterials-based delivery design have shown tremendous potential to provide clinically relevant solutions while overcoming several hurdles associated with current cancer immunotherapy technologies. Early implantable polymeric scaffolds that delivered tumor lysates, cytokines and adjuvants pioneered a shift in approach to vaccine administration as it enabled large amounts of DCs to be recruited to and primed for anti-tumor activity in situ. These scaffolds exhibited the potential to circumvent several hurdles faced by ex vivo activation of DC in vaccines such as the death of a significant population of the transplanted DCs or the two-patient procedure requirement which could be burdensome. Here, DCs are recruited and activated in one step, migrating directly to the target site to preserve a significant amount of cells that could be lost during transplantation.147 Since then, new designs have been developed to recruit DCs while bypassing the need for a surgical procedure. For instance, Kim et al. developed mesoporous silica rods with hexagonous mesoporous structures which could be injected through a needle then form macro-porous 3D structures in vivo that recruit and modulate DCs before the DCs traffic to their site of action. This design allows for the in situ assembly of the scaffold structure and has demonstrated the ability to recruit higher number of DCs than its predecessors.85
Smart biomaterial-based technologies that respond to biological or external stimuli such as pH, enzymatic activity, light, and temperature have been developed to improve control over cargo release, enhancing further the specificity of the drug delivered.191,192 pH-Responsive nanoparticles are one of the most prominent smart technologies used in cancer immunotherapy. These nanoparticles feature ionic polymers that change ionization state in response to pH in the endosome or tumor microenvironment, triggering either cytosolic release of cargo or selective release of cargo in the tumor microenvironment.193 Lately, multi-stimuli responsive delivery systems have been developed to enable even greater selectivity in drug delivery.192 Delivery platforms that respond to multiple stimuli have yet to be widely utilized for cancer immunotherapies. This serves a suitable opportunity to introduce more innovative drug delivery systems to the field of cancer immunotherapy that can target immunostimulatory drugs based on multiple stimuli relevant to the particular immune delivery challenge under investigation.
3.7 Clinical translation
The enhanced preclinical results achieved by immunostimulatory biomaterials has set the stage for many clinical trials involving these platforms. Biomaterial-based delivery systems have already been established as a viable method for delivering chemotherapy with myriads of clinical trials dedicated to biomaterial-loaded chemotherapies such as doxorubicin, paclitaxel and others. In the relatively newer field of cancer immunotherapy, clinical trials involving biomaterials have begun to emerge. A summary of phase I/II clinical trials for immunostimulatory biomaterials in can be found in Table 2. Most of these clinical trials are still ongoing therefore their results are pending, however some of these trials have exhibited promise. In one study, the polymer–cytokine conjugate PEG-IFN-α-2b achieved a median progression free and overall survival of 2 and 9.7 months respectively in patients with melanoma.194 As innovation continues to drive the wheels of cancer immunotherapy, more clinical trials involving sophisticated biomaterial-based designs are expected.| Biomaterial | Cancer type | Phase | Trial ID | Ref. |
|---|---|---|---|---|
| Liposome | Ovarian, breast and prostate cancer | I | NCT01095848 | 195 |
| Melanoma | I | NCT01052142 | 196 | |
| Melanoma | I | NCT02410733 | 197 | |
| Leukemia | I | NCT00860522 | 198 | |
| PEG-IFN-alfa (Intron) | Melanoma | II | NCT0049530 | 194 |
| Melanoma | I | NCT00457418 | 199 | |
| Melanoma | II | NCT01959633 | 200 | |
| Sacituzumab govitecan | Metastatic breast cancer | II | NCT04039230 | 201 |
| PLGA scaffold | Melanoma | I | NCT01753089 | 202 |
4. Conclusions
Cancer immunotherapy is a breakthrough form of cancer therapy with tremendous potential to generate treatment responses. The efficacy of cancer immunotherapy is however inhibited by immunosuppressive molecular pathways that render the tumor microenvironment poorly immunogenic and unresponsive to treatment. Novel strategies to boost tumor immunogenicity can overcome these suppressive pathways to attain desirable treatment responses. However, these strategies are attended with several adverse side-effects which include short half-life, poor cellular uptake, and off-target accumulation resulting in harmful activity in vivo. Local and systemic strategies that incorporate sophisticated biomaterials engineered to address these challenges show great potential to bring about proper therapeutic outcomes. Local strategies involve the use of scaffolds, hydrogels, and microparticles placed in the vicinity of the tumor or immune organs to locally release immune agonists and/or recruit immune cells in situ. Systemic strategies mediate drug delivery through nanoparticles and drug conjugates, among other technologies, in order to access disseminated sites such as hard-to-access tumors and metastases. Both local and systemic strategies utilize controlled or stimuli-responsive mechanisms to deliver agonists thereby improving the pharmacokinetics, biodistribution, and therapeutic window of the cargo. The biomaterials-based approaches to boost tumor immunogenicity that were reviewed here offer exciting new therapy directions with the potential to increase both the safety and efficacy of future cancer immunotherapies.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors would like to thank The Ronald E. McNair Program at The University of Mississippi for funding this research project though Grant #P217A170028 from the US Department of Education. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 1P20GM130460-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.References
- D. N. Khalil, E. L. Smith, R. J. Brentjens and J. D. Wolchok, Nat. Rev. Clin. Oncol., 2016, 13, 273–290 CrossRef CAS.
- J. Couzin-Frankel, Science, 2013, 342, 1432–1433 CrossRef CAS.
- A. J. Korman, K. S. Peggs and J. P. Allison, Adv. Immunol., 2007, 32 Search PubMed.
- P. C. Tumeh, C. L. Harview, J. H. Yearley, I. P. Shintaku, E. J. M. Taylor, L. Robert, B. Chmielowski, M. Spasic, G. Henry, V. Ciobanu, A. N. West, M. Carmona, C. Kivork, E. Seja, G. Cherry, A. J. Gutierrez, T. R. Grogan, C. Mateus, G. Tomasic, J. A. Glaspy, R. O. Emerson, H. Robins, R. H. Pierce, D. A. Elashoff, C. Robert and A. Ribas, Nature, 2014, 515, 568–571 CrossRef CAS.
- S. Wróbel, M. Przybyło and E. Stępień, J. Clin. Med., 2019, 8, 368 CrossRef.
- P. Fessas, H. Lee, S. Ikemizu and T. Janowitz, Semin. Oncol., 2017, 44, 136–140 CrossRef CAS.
- Q. Song, C. Zhang and X. Wu, Immunol. Lett., 2018, 196, 11–21 CrossRef CAS.
- R. Wang, W. Pan, L. Jin, W. Huang, Y. Li, D. Wu, C. Gao, D. Ma and S. Liao, Cancer Lett., 2020, 471, 88–102 CrossRef CAS.
- L. Jeanbart and M. A. Swartz, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 14467–14472 CrossRef CAS.
- R. M. Steinman, Annu. Rev. Immunol., 1991, 9(1), 271–296 CrossRef CAS.
- G. Parmiani, C. Castelli, P. Dalerba, R. Mortarini, L. Rivoltini, F. M. Marincola and A. Anichini, JNCI, J. Natl. Cancer Inst., 2002, 94, 805–818 CrossRef CAS.
- H. Kübler, B. Scheel, U. Gnad-Vogt, K. Miller, W. Schultze-Seemann, F. vom Dorp, G. Parmiani, C. Hampel, S. Wedel, L. Trojan, D. Jocham, T. Maurer, G. Rippin, M. Fotin-Mleczek, F. von der Mülbe, J. Probst, I. Hoerr, K.-J. Kallen, T. Lander and A. Stenzl, J. Immunother. Cancer, 2015, 3, 26 CrossRef.
- L. Chen, D. Qiao, J. Wang, G. Tian and M. Wang, Immunol. Lett., 2019, 216, 51–62 CrossRef CAS.
- C. H. June, R. S. O'Connor, O. U. Kawalekar, S. Ghassemi and M. C. Milone, Science, 2018, 359, 1361–1365 CrossRef CAS.
- T. Karpanen and J. Olweus, Mol. Oncol., 2015, 9, 2019–2042 CrossRef CAS.
- V. Sathyanarayanan and S. S. Neelapu, Mol. Oncol., 2015, 9, 2043–2053 CrossRef CAS.
- P. Sharma and J. P. Allison, Science, 2015, 348, 56–61 CrossRef CAS.
- P. Bonaventura, T. Shekarian, V. Alcazer, J. Valladeau-Guilemond, S. Valsesia-Wittmann, S. Amigorena, C. Caux and S. Depil, Front. Immunol., 2019, 10, 168 CrossRef CAS.
- W. Zou, Nat. Rev. Immunol., 2006, 6, 295–307 CrossRef CAS.
- H. Nishikawa and S. Sakaguchi, Curr. Opin. Immunol., 2014, 27, 1–7 CrossRef CAS.
- D. A. A. Vignali, L. W. Collison and C. J. Workman, Nat. Rev. Immunol., 2008, 8, 523–532 CrossRef CAS.
- D. M. S. Hossain, A. K. Panda, A. Manna, S. Mohanty, P. Bhattacharjee, S. Bhattacharyya, T. Saha, S. Chakraborty, R. K. Kar, T. Das, S. Chatterjee and G. Sa, Immunity, 2013, 39, 1057–1069 CrossRef CAS.
- K. L. Dennis, N. R. Blatner, F. Gounari and K. Khazaie, Curr. Opin. Oncol., 2013, 25, 637–645 CrossRef CAS.
- A. G. Jarnicki, J. Lysaght, S. Todryk and K. H. G. Mills, J. Immunol., 2006, 177, 896–904 CrossRef CAS.
- M. O. Li, Y. Y. Wan, S. Sanjabi, A.-K. L. Robertson and R. A. Flavell, Annu. Rev. Immunol., 2006, 24, 99–146 CrossRef CAS.
- N. Larmonier, M. Marron, Y. Zeng, J. Cantrell, A. Romanoski, M. Sepassi, S. Thompson, X. Chen, S. Andreansky and E. Katsanis, Cancer Immunol. Immunother., 2006, 56, 48–59 CrossRef.
- M.-L. Chen, M. J. Pittet, L. Gorelik, R. A. Flavell, R. Weissleder, H. von Boehmer and K. Khazaie, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 419–424 CrossRef CAS.
- O. S. Qureshi, Y. Zheng, K. Nakamura, K. Attridge, C. Manzotti, E. M. Schmidt, J. Baker, L. E. Jeffery, S. Kaur, Z. Briggs, T. Z. Hou, C. E. Futter, G. Anderson, L. S. K. Walker and D. M. Sansom, Science, 2011, 332, 600–603 CrossRef CAS.
- M. J. Selby, J. J. Engelhardt, M. Quigley, K. A. Henning, T. Chen, M. Srinivasan and A. J. Korman, Cancer Immunol. Res., 2013, 1, 32–42 CrossRef CAS.
- B. Sun, M. Liu, M. Cui and T. Li, Immunol. Lett., 2020, 217, 7–14 CrossRef CAS.
- X. Cao, S. F. Cai, T. A. Fehniger, J. Song, L. I. Collins, D. R. Piwnica-Worms and T. J. Ley, Immunity, 2007, 27, 635–646 CrossRef CAS.
- S. P. Cullen, M. Brunet and S. J. Martin, Cell Death Differ., 2010, 17, 616–623 CrossRef CAS.
- P. Pandiyan, L. Zheng, S. Ishihara, J. Reed and M. J. Lenardo, Nat. Immunol., 2007, 8, 1353–1362 CrossRef CAS.
- M. D. Sharma, R. Shinde, T. L. McGaha, L. Huang, R. B. Holmgaard, J. D. Wolchok, M. R. Mautino, E. Celis, A. H. Sharpe, L. M. Francisco, J. D. Powell, H. Yagita, A. L. Mellor, B. R. Blazar and D. H. Munn, Sci. Adv., 2015, 1, e1500845 CrossRef.
- D. H. Munn, M. D. Sharma, T. S. Johnson and P. Rodriguez, Cancer Immunol. Immunother., 2017, 66, 1049–1058 CrossRef CAS.
- R. Noy and J. W. Pollard, Immunity, 2014, 41, 49–61 CrossRef CAS.
- E. Y. Lin, J.-F. Li, L. Gnatovskiy, Y. Deng, L. Zhu, D. A. Grzesik, H. Qian, X.-N. Xue and J. W. Pollard, Cancer Res., 2006, 66, 11238–11246 CrossRef CAS.
- J.-Y. Shih, A. Yuan, J. J. W. Chen and P. C. Yang, J. Cancer Mol., 2(3), 101–106 CAS.
- J. Chen, Y. Yao, C. Gong, F. Yu, S. Su, J. Chen, B. Liu, H. Deng, F. Wang, L. Lin, H. Yao, F. Su, K. S. Anderson, Q. Liu, M. E. Ewen, X. Yao and E. Song, Cancer Cell, 2011, 19, 541–555 CrossRef CAS.
- S. K. Biswas, P. Allavena and A. Mantovani, Semin. Immunopathol., 2013, 35, 585–600 CrossRef CAS.
- J. G. Quatromoni and E. Eruslanov, Am. J. Transl. Res., 2012, 4(4), 376 Search PubMed.
- S. Gordon, Nat. Rev. Immunol., 2003, 3, 23–35 CrossRef CAS.
- K. A. Jablonski, S. A. Amici, L. M. Webb, J. de D. Ruiz-Rosado, P. G. Popovich, S. Partida-Sanchez and M. Guerau-de-Arellano, PLoS One, 2015, 10, e0145342 CrossRef.
- T. A. Werfel and R. S. Cook, Semin. Immunopathol., 2018, 40, 545–554 CrossRef.
- T. A. Werfel, D. L. Elion, B. Rahman, D. J. Hicks, V. Sanchez, P. I. Gonzales-Ericsson, M. J. Nixon, J. L. James, J. M. Balko, P. A. Scherle, H. K. Koblish and R. S. Cook, Cancer Res., 2019, 79, 171–182 CrossRef CAS.
- M. L. Sulciner, C. N. Serhan, M. M. Gilligan, D. K. Mudge, J. Chang, A. Gartung, K. A. Lehner, D. R. Bielenberg, B. Schmidt, J. Dalli, E. R. Greene, Y. Gus-Brautbar, J. Piwowarski, T. Mammoto, D. Zurakowski, M. Perretti, V. P. Sukhatme, A. Kaipainen, M. W. Kieran, S. Huang and D. Panigrahy, J. Exp. Med., 2018, 215, 115–140 CrossRef CAS.
- D. R. Korns, S. C. Frasch, R. Fernandez-Boyanapalli, P. M. Henson and D. L. Bratton, Front. Immunol., 2011, 2, 57 Search PubMed.
- G. C. Prendergast, A. Mondal, S. Dey, L. D. Laury-Kleintop and A. J. Muller, Trends Cancer, 2018, 4, 38–58 CrossRef CAS.
- T. Voron, E. Marcheteau, S. Pernot, O. Colussi, E. Tartour, J. Taieb and M. Terme, Front. Oncol., 2014, 4, 70 Search PubMed.
- M. Kudo, Cancer, 2020, 12, 1089 CrossRef.
- H. Kato, O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C.-S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita and S. Akira, Nature, 2006, 441, 101–105 CrossRef CAS.
- D. L. Elion, M. E. Jacobson, D. J. Hicks, B. Rahman, V. Sanchez, P. I. Gonzales-Ericsson, O. Fedorova, A. M. Pyle, J. T. Wilson and R. S. Cook, Cancer Res., 2018, 78, 6183–6195 CrossRef CAS.
- D. L. Elion and R. S. Cook, Oncotarget, 2018, 9(48), 29007 CrossRef.
- C. R. Palmer, M. E. Jacobson, O. Fedorova, A. M. Pyle and J. T. Wilson, Bioconjugate Chem., 2018, 29, 742–747 CrossRef CAS.
- M. E. Jacobson, L. Wang-Bishop, K. W. Becker and J. T. Wilson, Biomater. Sci., 2019, 7, 547–559 RSC.
- G. N. Barber, Nat. Rev. Immunol., 2015, 15, 760–770 CrossRef CAS.
- D. Shae, K. W. Becker, P. Christov, D. S. Yun, A. K. R. Lytton-Jean, S. Sevimli, M. Ascano, M. Kelley, D. B. Johnson, J. M. Balko and J. T. Wilson, Nat. Nanotechnol., 2019, 14, 269–278 CrossRef CAS.
- L. Wang-Bishop, M. Wehbe, D. Shae, J. James, B. C. Hacker, K. Garland, P. P. Chistov, M. Rafat, J. M. Balko and J. T. Wilson, J. Immunother. Cancer, 2020, 8, e000282 CrossRef.
- S.-R. Woo, M. B. Fuertes, L. Corrales, S. Spranger, M. J. Furdyna, M. Y. K. Leung, R. Duggan, Y. Wang, G. N. Barber, K. A. Fitzgerald, M.-L. Alegre and T. F. Gajewski, Immunity, 2014, 41, 830–842 CrossRef CAS.
- D. M. Kasumba and N. Grandvaux, Trends Pharmacol. Sci., 2019, 40, 116–127 CrossRef CAS.
- J. Daßler-Plenker, A. Paschen, B. Putschli, S. Rattay, S. Schmitz, M. Goldeck, E. Bartok, G. Hartmann and C. Coch, Int. J. Cancer, 2019, 144, 1645–1656 CrossRef.
- T. Zillinger and G. Hartmann, Mol. Ther., 2019, 27, 491–492 CrossRef CAS.
- D. R. Ranoa, A. D. Parekh, S. P. Pitroda, X. Huang, T. Darga, A. C. Wong, L. Huang, J. Andrade, J. P. Staley, T. Satoh, S. Akira, R. R. Weichselbaum and N. N. Khodarev, Oncotarget, 2016, 7, 26496–26515 CrossRef.
- S. Sharma, I. Garg and M. Z. Ashraf, Vasc. Pharmacol., 2016, 87, 30–37 CrossRef CAS.
- G. K. Gupta and D. K. Agrawal, BioDrugs, 2010, 24, 225–235 CrossRef CAS.
- H. Kim, L. Niu, P. Larson, T. A. Kucaba, K. A. Murphy, B. R. James, D. M. Ferguson, T. S. Griffith and J. Panyam, Biomaterials, 2018, 164, 38–53 CrossRef CAS.
- H. Zhang, T. Yan, S. Xu, S. Feng, D. Huang, M. Fujita and X.-D. Gao, Mater. Sci. Eng., C, 2017, 73, 144–151 CrossRef.
- H. Shirota and D. M. Klinman, Expert Rev. Vaccines, 2014, 13, 299–312 CrossRef CAS.
- L. E. Kandalaft, N. Singh, J. B. Liao, A. Facciabene, J. S. Berek, D. J. Powell and G. Coukos, Gynecol. Oncol., 2010, 116, 222–233 CrossRef CAS.
- G. D'Errico, H. L. Machado and B. Sainz, Clin. Transl. Med., 2017, 6(1), 1–10 Search PubMed.
- S. J. Oiseth and M. S. Aziz, J. Cancer Metastasis Treat., 2017, 3, 250 CrossRef CAS.
- M. O. Dellacherie, B. R. Seo and D. J. Mooney, Nat. Rev. Mater., 2019, 4, 379–397 CrossRef.
- C. J. Kearney and D. J. Mooney, Nat. Mater., 2013, 12, 1004–1017 CrossRef CAS.
- P. Huang, X. Wang, X. Liang, J. Yang, C. Zhang, D. Kong and W. Wang, Acta Biomater., 2019, 85, 1–26 CrossRef CAS.
- Q. Chai, Y. Jiao and X. Yu, Gels, 2017, 3, 6 CrossRef.
- A. Chyzy, M. Tomczykowa and M. E. Plonska-Brzezinska, Materials, 2020, 13, 188 CrossRef CAS.
- N. A. Peppas, Y. Huang, M. Torres-Lugo, J. H. Ward and J. Zhang, Annu. Rev. Biomed. Eng., 2000, 2, 9–29 CrossRef CAS.
- C. M. Kirschner and K. S. Anseth, Acta Mater., 2013, 61, 931–944 CrossRef CAS.
- H. K. Lau and K. L. Kiick, Biomacromolecules, 2015, 16, 28–42 CrossRef CAS.
- P. M. Kharkar, K. L. Kiick and A. M. Kloxin, Chem. Soc. Rev., 2013, 42, 7335–7372 RSC.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1880 CrossRef CAS.
- T. Vermonden, R. Censi and W. E. Hennink, Chem. Rev., 2012, 112, 2853–2888 CrossRef CAS.
- J. Yan, Y. Miao, H. Tan, T. Zhou, Z. Ling, Y. Chen, X. Xing and X. Hu, Mater. Sci. Eng., C, 2016, 63, 274–284 CrossRef CAS.
- S. T. Koshy, T. C. Ferrante, S. A. Lewin and D. J. Mooney, Biomaterials, 2014, 35, 2477–2487 CrossRef CAS.
- J. Kim, W. A. Li, Y. Choi, S. A. Lewin, C. S. Verbeke, G. Dranoff and D. J. Mooney, Nat. Biotechnol., 2015, 33, 64–72 CrossRef CAS.
- A. S. Cheung, D. K. Y. Zhang, S. T. Koshy and D. J. Mooney, Nat. Biotechnol., 2018, 36, 160–169 CrossRef CAS.
- B. Subia, J. Kundu and S. C. Kundu, in Tissue Engineering, ed. D. Eberli, InTech, 2010 Search PubMed.
- Y. Yang, X. Qiao, R. Huang, H. Chen, X. Shi, J. Wang, W. Tan and Z. Tan, Biomaterials, 2020, 230, 119618 CrossRef CAS.
- W. A. Li, B. Y. Lu, L. Gu, Y. Choi, J. Kim and D. J. Mooney, Biomaterials, 2016, 83, 249–256 CrossRef CAS.
- C. Foged, B. Brodin, S. Frokjaer and A. Sundblad, Int. J. Pharm., 2005, 298, 315–322 CrossRef CAS.
- J. J. Moon, B. Huang and D. J. Irvine, Adv. Mater., 2012, 24, 3724–3746 CrossRef CAS.
- S. Lee and K. Margolin, Cancer, 2011, 3, 3856–3893 CrossRef CAS.
- O. Hamid, C. Robert, A. Daud, F. S. Hodi, W.-J. Hwu, R. Kefford, J. D. Wolchok, P. Hersey, R. W. Joseph, J. S. Weber, R. Dronca, T. C. Gangadhar, A. Patnaik, H. Zarour, A. M. Joshua, K. Gergich, J. Elassaiss-Schaap, A. Algazi, C. Mateus, P. Boasberg, P. C. Tumeh, B. Chmielowski, S. W. Ebbinghaus, X. N. Li, S. P. Kang and A. Ribas, N. Engl. J. Med., 2013, 369, 134–144 CrossRef CAS.
- S. L. Topalian, F. S. Hodi, J. R. Brahmer, S. N. Gettinger, D. C. Smith, D. F. McDermott, J. D. Powderly, R. D. Carvajal, J. A. Sosman, M. B. Atkins, P. D. Leming, D. R. Spigel, S. J. Antonia, L. Horn, C. G. Drake, D. M. Pardoll, L. Chen, W. H. Sharfman, R. A. Anders, J. M. Taube, T. L. McMiller, H. Xu, A. J. Korman, M. Jure-Kunkel, S. Agrawal, D. McDonald, G. D. Kollia, A. Gupta, J. M. Wigginton and M. Sznol, N. Engl. J. Med., 2012, 366, 2443–2454 CrossRef CAS.
- M. Hussein, J. R. Berenson, R. Niesvizky, N. Munshi, J. Matous, R. Sobecks, K. Harrop, J. G. Drachman and N. Whiting, Haematologica, 2010, 95, 845–848 CrossRef CAS.
- D. S. Pisal, M. P. Kosloski and S. V. Balu-Iyer, J. Pharm. Sci., 2010, 99, 2557–2575 CrossRef CAS.
- A. S. Cheung and D. J. Mooney, Nano Today, 2015, 10, 511–531 CrossRef CAS.
- M. Graciotti, C. Berti, H.-A. Klok and L. Kandalaft, J. Transl. Med., 2017, 15, 142 CrossRef.
- V. Torchilin, Adv. Drug Delivery Rev., 2011, 63, 131–135 CrossRef CAS.
- R. Duncan, Nat. Rev. Drug Discovery, 2003, 2, 347–360 CrossRef CAS.
- Y. Matsumoto, J. W. Nichols, K. Toh, T. Nomoto, H. Cabral, Y. Miura, R. J. Christie, N. Yamada, T. Ogura, M. R. Kano, Y. Matsumura, N. Nishiyama, T. Yamasoba, Y. H. Bae and K. Kataoka, Nat. Nanotechnol., 2016, 11, 533–538 CrossRef CAS.
- S. Sindhwani, A. M. Syed, J. Ngai, B. R. Kingston, L. Maiorino, J. Rothschild, P. MacMillan, Y. Zhang, N. U. Rajesh, T. Hoang, J. L. Y. Wu, S. Wilhelm, A. Zilman, S. Gadde, A. Sulaiman, B. Ouyang, Z. Lin, L. Wang, M. Egeblad and W. C. W. Chan, Nat. Mater., 2020, 19, 566–575 CrossRef CAS.
- A. M. Sofias, Y. C. Toner, A. E. Meerwaldt, M. M. T. van Leent, G. Soultanidis, M. Elschot, H. Gonai, K. Grendstad, Å. Flobak, U. Neckmann, C. Wolowczyk, E. L. Fisher, T. Reiner, C. de L. Davies, G. Bjørkøy, A. J. P. Teunissen, J. Ochando, C. Pérez-Medina, W. J. M. Mulder and S. Hak, ACS Nano, 2020, 14(7), 7832–7846 CrossRef CAS.
- S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer and W. C. W. Chan, Nano Lett., 2009, 9, 1909–1915 CrossRef CAS.
- F. Alexis, E. Pridgen, L. K. Molnar and O. C. Farokhzad, Mol. Pharm., 2008, 5, 505–515 CrossRef CAS.
- P. T. Wong and S. K. Choi, Chem. Rev., 2015, 115, 3388–3432 CrossRef CAS.
- C. B. Rodell, S. P. Arlauckas, M. F. Cuccarese, C. S. Garris, R. Li, M. S. Ahmed, R. H. Kohler, M. J. Pittet and R. Weissleder, Nat. Biomed. Eng., 2018, 2, 578–588 CrossRef CAS.
- M. A. Miller, R. Chandra, M. F. Cuccarese, C. Pfirschke, C. Engblom, S. Stapleton, U. Adhikary, R. H. Kohler, J. F. Mohan, M. J. Pittet and R. Weissleder, Sci. Transl. Med., 2017, 9, eaal0225 CrossRef.
- M. A. Miller, S. Gadde, C. Pfirschke, C. Engblom, M. M. Sprachman, R. H. Kohler, K. S. Yang, A. M. Laughney, G. Wojtkiewicz, N. Kamaly, S. Bhonagiri, M. J. Pittet, O. C. Farokhzad and R. Weissleder, Sci. Transl. Med., 2015, 7, 314ra183 CrossRef.
- S. J. Wang, R. Li, T. S. C. Ng, G. Luthria, M. J. Oudin, M. Prytyskach, R. H. Kohler, R. Weissleder, D. A. Lauffenburger and M. A. Miller, Sci. Adv., 2020, 6, eaaz8521 CrossRef.
- M. A. Miller, Y.-R. Zheng, S. Gadde, C. Pfirschke, H. Zope, C. Engblom, R. H. Kohler, Y. Iwamoto, K. S. Yang, B. Askevold, N. Kolishetti, M. Pittet, S. J. Lippard, O. C. Farokhzad and R. Weissleder, Nat. Commun., 2015, 6, 8692 CrossRef CAS.
- P. Korangath, J. D. Barnett, A. Sharma, E. T. Henderson, J. Stewart, S.-H. Yu, S. K. Kandala, C.-T. Yang, J. S. Caserto, M. Hedayati, T. D. Armstrong, E. Jaffee, C. Gruettner, X. C. Zhou, W. Fu, C. Hu, S. Sukumar, B. W. Simons and R. Ivkov, Sci. Adv., 2020, 6, eaay1601 CrossRef.
- P. D. Senter, Curr. Opin. Chem. Biol., 2009, 13, 235–244 CrossRef CAS.
- B. A. Teicher and R. V. J. Chari, Clin. Cancer Res., 2011, 17, 6389–6397 CrossRef CAS.
- H. L. Perez, P. M. Cardarelli, S. Deshpande, S. Gangwar, G. M. Schroeder, G. D. Vite and R. M. Borzilleri, Drug Discovery Today, 2014, 19, 869–881 CrossRef CAS.
- E. L. Sievers and P. D. Senter, Annu. Rev. Med., 2013, 64, 15–29 CrossRef CAS.
- I. Ekladious, Y. L. Colson and M. W. Grinstaff, Nat. Rev. Drug Discovery, 2019, 18, 273–294 CrossRef CAS.
- P. Caliceti, Adv. Drug Delivery Rev., 2003, 55, 1261–1277 CrossRef CAS.
- P. L. Turecek, M. J. Bossard, F. Schoetens and I. A. Ivens, J. Pharm. Sci., 2016, 105, 460–475 CrossRef CAS.
- D. G. Leach, S. Young and J. D. Hartgerink, Acta Biomater., 2019, 88, 15–31 CrossRef CAS.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS.
- Y. Liu, L. Xiao, K.-I. Joo, B. Hu, J. Fang and P. Wang, Biomacromolecules, 2014, 15, 3836–3845 CrossRef CAS.
- G. W. Bos, J. J. L. Jacobs, J. W. Koten, S. Van Tomme, T. Veldhuis, C. F. van Nostrum, W. Den Otter and W. E. Hennink, Eur. J. Pharm. Sci., 2004, 21, 561–567 CrossRef CAS.
- K. Xu, F. Lee, S. J. Gao, J. E. Chung, H. Yano and M. Kurisawa, J. Controlled Release, 2013, 166, 203–210 CrossRef CAS.
- S. Ishii, J. Kaneko and Y. Nagasaki, Biomaterials, 2016, 84, 210–218 CrossRef CAS.
- H. Song, P. Huang, J. Niu, G. Shi, C. Zhang, D. Kong and W. Wang, Biomaterials, 2018, 159, 119–129 CrossRef CAS.
- Q. Zhan, B. Shen, Y. Fang, X. Deng, H. Chen, J. Jin, C. Peng and H. Li, Colloids Surf., B, 2017, 158, 469–473 CrossRef CAS.
- T. Hemmerle and D. Neri, Int. J. Cancer, 2014, 134, 467–477 CrossRef.
- C. Hess and D. Neri, Cancer Immunol. Immunother., 2015, 64, 635–644 CrossRef CAS.
- W. Sun, W. Ji, Q. Hu, J. Yu, C. Wang, C. Qian, G. Hochu and Z. Gu, Biomaterials, 2016, 96, 1–10 CrossRef CAS.
- Y. Wang, Y.-X. Lin, S.-L. Qiao, H.-W. An, Y. Ma, Z.-Y. Qiao, R. P. Y. J. Rajapaksha and H. Wang, Biomaterials, 2017, 112, 153–163 CrossRef CAS.
- D. G. Leach, N. Dharmaraj, S. L. Piotrowski, T. L. Lopez-Silva, Y. L. Lei, A. G. Sikora, S. Young and J. D. Hartgerink, Biomaterials, 2018, 163, 67–75 CrossRef CAS.
- C. G. Park, C. A. Hartl, D. Schmid, E. M. Carmona, H.-J. Kim and M. S. Goldberg, Sci. Transl. Med., 2018, 10, eaar1916 CrossRef.
- T. T. Smith, H. F. Moffett, S. B. Stephan, C. F. Opel, A. G. Dumigan, X. Jiang, V. G. Pillarisetty, S. P. S. Pillai, K. D. Wittrup and M. T. Stephan, J. Clin. Invest., 2017, 127, 2176–2191 CrossRef.
- N. Cheng, R. Watkins-Schulz, R. D. Junkins, C. N. David, B. M. Johnson, S. A. Montgomery, K. J. Peine, D. B. Darr, H. Yuan, K. P. McKinnon, Q. Liu, L. Miao, L. Huang, E. M. Bachelder, K. M. Ainslie and J. P.-Y. Ting, JCI Insight, 2018, 3, e120638 CrossRef.
- S. T. Koshy, A. S. Cheung, L. Gu, A. R. Graveline and D. J. Mooney, Adv. Biosyst., 2017, 1, 1600013 CrossRef.
- T. Nakamura, H. Miyabe, M. Hyodo, Y. Sato, Y. Hayakawa and H. Harashima, J. Controlled Release, 2015, 216, 149–157 CrossRef CAS.
- C. M. Jewell, S. C. Bustamante Lopez and D. J. Irvine, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 15745–15750 CrossRef CAS.
- D. R. Wilson, R. Sen, J. C. Sunshine, D. M. Pardoll, J. J. Green and Y. J. Kim, Nanomedicine, 2018, 14, 237–246 CrossRef CAS.
- H. T. T. Duong, T. Thambi, Y. Yin, S. H. Kim, T. L. Nguyen, V. H. G. Phan, J. Kim, J. H. Jeong and D. S. Lee, Biomaterials, 2020, 230, 119599 CrossRef CAS.
- H. Song, P. Yang, P. Huang, C. Zhang, D. Kong and W. Wang, Theranostics, 2019, 9, 2299–2314 CrossRef CAS.
- V. B. Joshi, S. M. Geary and A. K. Salem, AAPS J., 2013, 15, 85–94 CrossRef CAS.
- P. Pradhan, H. Qin, J. A. Leleux, D. Gwak, I. Sakamaki, L. W. Kwak and K. Roy, Biomaterials, 2014, 35, 5491–5504 CrossRef CAS.
- J. Park, S. H. Wrzesinski, E. Stern, M. Look, J. Criscione, R. Ragheb, S. M. Jay, S. L. Demento, A. Agawu, P. Licona Limon, A. F. Ferrandino, D. Gonzalez, A. Habermann, R. A. Flavell and T. M. Fahmy, Nat. Mater., 2012, 11, 895–905 CrossRef CAS.
- Y. Zhang, N. Li, H. Suh and D. J. Irvine, Nat. Commun., 2018, 9, 6 CrossRef.
- F. Zhang, S. B. Stephan, C. I. Ene, T. T. Smith, E. C. Holland and M. T. Stephan, Cancer Res., 2018, 78(13), 3718–3730 CAS.
- O. A. Ali, N. Huebsch, L. Cao, G. Dranoff and D. J. Mooney, Nat. Mater., 2009, 8, 151–158 CrossRef CAS.
- O. A. Ali, P. Tayalia, D. Shvartsman, S. Lewin and D. J. Mooney, Adv. Funct. Mater., 2013, 23, 4621–4628 CrossRef CAS.
- A. Ramesh, S. Kumar, D. Nandi and A. Kulkarni, Adv. Mater., 2019, 31, 1904364 CrossRef CAS.
- F. Zhang, N. N. Parayath, C. I. Ene, S. B. Stephan, A. L. Koehne, M. E. Coon, E. C. Holland and M. T. Stephan, Nat. Commun., 2019, 10, 3974 CrossRef CAS.
- S. Foster, C. L. Duvall, E. F. Crownover, A. S. Hoffman and P. S. Stayton, Bioconjugate Chem., 2010, 21, 2205–2212 CrossRef CAS.
- F. Qiu, K. W. Becker, F. C. Knight, J. J. Baljon, S. Sevimli, D. Shae, P. Gilchuk, S. Joyce and J. T. Wilson, Biomaterials, 2018, 182, 82–91 CrossRef CAS.
- H. Shen, A. L. Ackerman, V. Cody, A. Giodini, E. R. Hinson, P. Cresswell, R. L. Edelson, W. M. Saltzman and D. J. Hanlon, Immunology, 2006, 117, 78–88 CrossRef CAS.
- J. C. Sunshine, K. Perica, J. P. Schneck and J. J. Green, Biomaterials, 2014, 35, 269–277 CrossRef CAS.
- S. Song, X. Jin, L. Zhang, C. Zhao, Y. Ding, Q. Ang, O. Khaidav and C. Shen, Int. J. Nanomed., 2019, 14, 2465–2483 CrossRef CAS.
- E. R. Steenblock and T. M. Fahmy, Mol. Ther., 2008, 16, 765–772 CrossRef CAS.
- P. M. Nguyen and T. L. Putoczki, Cytokine, 2019, 118, 8–18 CrossRef CAS.
- S. Liang, Z. Chen, G. Jiang, Y. Zhou, Q. Liu, Q. Su, W. Wei, J. Du and H. Wang, Cancer Lett., 2017, 386, 12–23 CrossRef CAS.
- T. L. Whiteside, Oncogene, 2008, 27, 5904–5912 CrossRef CAS.
- M. Dougan, J. R. Ingram, H.-J. Jeong, M. M. Mosaheb, P. T. Bruck, L. Ali, N. Pishesha, O. Blomberg, P. M. Tyler, M. M. Servos, M. Rashidian, Q.-D. Nguyen, U. H. von Andrian, H. L. Ploegh and S. K. Dougan, Cancer Immunol. Res., 2018, 6, 389–401 CrossRef CAS.
- E. Ortiz-Sánchez, G. Helguera, T. R. Daniels and M. L. Penichet, Expert Opin. Biol. Ther., 2008, 8, 609–632 CrossRef.
- P. Hu, M. Mizokami, G. Ruoff, L. A. Khawli and A. L. Epstein, Blood, 2003, 101, 4853–4861 CrossRef CAS.
- S. Létourneau, E. M. M. van Leeuwen, C. Krieg, C. Martin, G. Pantaleo, J. Sprent, C. D. Surh and O. Boyman, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 2171–2176 CrossRef.
- T. Floros and A. A. Tarhini, Semin. Oncol., 2015, 42, 539–548 CrossRef CAS.
- C. Halin, V. Gafner, M. E. Villani, L. Borsi, A. Berndt, H. Kosmehl, L. Zardi and D. Neri, Cancer Res., 2003, 6312, 3202–3210 Search PubMed.
- C. Hutmacher and D. Neri, Adv. Drug Delivery Rev., 2019, 141, 67–91 CrossRef CAS.
- R. E. Kontermann, Arch. Biochem. Biophys., 2012, 526, 194–205 CrossRef CAS.
- P. Probst, J. Kopp, A. Oxenius, M. P. Colombo, D. Ritz, T. Fugmann and D. Neri, Cancer Res., 2017, 77, 3644–3654 CrossRef CAS.
- C. Hess and D. Neri, Exp. Biol. Med., 2014, 239, 842–852 CrossRef.
- G. Shim, M.-G. Kim, J. Y. Park and Y.-K. Oh, Asian J. Pharm. Sci., 2013, 8, 72–80 CrossRef CAS.
- R. Zhang, M. M. Billingsley and M. J. Mitchell, J. Controlled Release, 2018, 292, 256–276 CrossRef CAS.
- M. Morishita, Y. Takahashi, A. Matsumoto, M. Nishikawa and Y. Takakura, Biomaterials, 2016, 111, 55–65 CrossRef CAS.
- G. Du, R. M. Hathout, M. Nasr, M. R. Nejadnik, J. Tu, R. I. Koning, A. J. Koster, B. Slütter, A. Kros, W. Jiskoot, J. A. Bouwstra and J. Mönkäre, J. Controlled Release, 2017, 266, 109–118 CrossRef CAS.
- J. M. Silva, E. Zupancic, G. Vandermeulen, V. G. Oliveira, A. Salgado, M. Videira, M. Gaspar, L. Graca, V. Préat and H. F. Florindo, J. Controlled Release, 2015, 198, 91–103 CrossRef CAS.
- J. T. Wilson, S. Keller, M. J. Manganiello, C. Cheng, C.-C. Lee, C. Opara, A. Convertine and P. S. Stayton, ACS Nano, 2013, 7, 3912–3925 CrossRef CAS.
- Y.-Q. Xie, L. Wei and L. Tang, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2018, 10, e1506 Search PubMed.
- S. Kumar, S. S. Kesharwani, B. Kuppast, M. A. Bakkari and H. Tummala, J. Controlled Release, 2017, 261, 263–274 CrossRef CAS.
- W. Song, S. N. Musetti and L. Huang, Biomaterials, 2017, 148, 16–30 CrossRef CAS.
- L. K. Petersen, A. E. Ramer-Tait, S. R. Broderick, C.-S. Kong, B. D. Ulery, K. Rajan, M. J. Wannemuehler and B. Narasimhan, Biomaterials, 2011, 32, 6815–6822 CrossRef CAS.
- N. M. Molino, M. Neek, J. A. Tucker, E. L. Nelson and S.-W. Wang, Biomaterials, 2016, 86, 83–91 CrossRef CAS.
- J. A. Rosenthal, L. Chen, J. L. Baker, D. Putnam and M. P. DeLisa, Curr. Opin. Biotechnol., 2014, 28, 51–58 CrossRef CAS.
- Y. Lin, J. Xu and H. Lan, J. Hematol. Oncol., 2019, 12, 76 CrossRef.
- Y. Zhu, B. L. Knolhoff, M. A. Meyer, T. M. Nywening, B. L. West, J. Luo, A. Wang-Gillam, S. P. Goedegebuure, D. C. Linehan and D. G. DeNardo, Cancer Res., 2014, 74, 5057–5069 CrossRef CAS.
- L. Liu, H. Yi, H. He, H. Pan, L. Cai and Y. Ma, Biomaterials, 2017, 134, 166–179 CrossRef CAS.
- N. N. Parayath, A. Parikh and M. M. Amiji, Nano Lett., 2018, 18, 3571–3579 CrossRef CAS.
- M.-L. De Temmerman, J. Rejman, J. Demeester, D. J. Irvine, B. Gander and S. C. De Smedt, Drug Discovery Today, 2011, 16, 569–582 CrossRef CAS.
- J. Koerner, D. Horvath and M. Groettrup, Front. Immunol., 2019, 10, 707 CrossRef CAS.
- Y.-W. Yang and P. Y.-J. Hsu, Biomaterials, 2008, 29, 2516–2526 CrossRef CAS.
- J. C. Sunshine and J. J. Green, Nanomedicine, 2013, 8, 1173–1189 CrossRef CAS.
- L. J. Eggermont, L. E. Paulis, J. Tel and C. G. Figdor, Trends Biotechnol., 2014, 32, 456–465 CrossRef CAS.
- Y. Lu, A. A. Aimetti, R. Langer and Z. Gu, Nat. Rev. Mater., 2017, 2, 16075 CrossRef CAS.
- P. S. Kowalski, C. Bhattacharya, S. Afewerki and R. Langer, ACS Biomater. Sci. Eng., 2018, 4, 3809–3817 CrossRef CAS.
- M. Kanamala, W. R. Wilson, M. Yang, B. D. Palmer and Z. Wu, Biomaterials, 2016, 85, 152–167 CrossRef CAS.
- U.S. National Library of Medicine, PEG-Interferon Alfa-2b in Treating Patients With Stage IV Melanoma, 2015, https://clinicaltrials.gov/ct2/show/results/NCT0049530?term=PEG&cond=MELANOMA&draw=2&rank=7 Search PubMed.
- U.S. National Library of Medicine, A Phase I Safety Study of a Cancer Vaccine to Treat HLA-A2 Positive Advanced Stage Ovarian, Breast and Prostate Cancer, 2015, http://clinicaltrials.gov/ct2/show/NCT01095848 Search PubMed.
- U.S. National Library of Medicine, Safety Study of a Liposome Vaccine to Treat Malignant Melanoma, 2012, http://clinicaltrials.gov/ct2/show/record/NCT01052142 Search PubMed.
- U.S. National Library of Medicine, Evaluation of the Safety and Tolerability of i.v. Administration of a Cancer Vaccine in Patients with Advanced Melanoma (Lipo-MERIT), 2020, https://clinicaltrials.gov/ct2/show/NCT02410733?cond=NCT02410733&draw=2&rank=1 Search PubMed.
- U.S. National Library of Medicine, JVRS-100 for the Treatment of Patients with relapsed or Refractory Leukemia, 2019, http://clinicaltrials.gov/ct2/show/record/NCT00860522 Search PubMed.
- U.S. National Library of Medicine, High-Dose PEG-intron Pharmacokinetic Study in Patients With Melanoma (Study P04831 AM2), 2017, http://clinicaltrials.gov/ct2/show/results/NCT00457418?term=PEG&cond=MELANOMA&draw=2&rank=4 Search PubMed.
- U.S. National Library of Medicine, Vemurafenib Plus Cobimetinib Plus PEG-interferon in Advanced Melanoma Patients Harboring the V600BRAF Mutation (VEMUPLINT), 2019, http://clinicaltrials.gov/show/NCT01959633?term=PEG&cond=MELANOMA&draw=2&rank=3 Search PubMed.
- U.S. National Library of Medicine, Study to Evaluate Sacituzumab Govitecan in Combination With Talazoparib in Patients With Metastatic Breast Cancer, 2020, http://clinicaltrials.gov/ct2/show/record/NCT04039230?cond=Breast+Cancer&draw=3&rank=12 Search PubMed.
- U.S. National Library of Medicine, Dendritic Cell Activating Scaffold in Melanoma, 2020, http://clinicaltrials.gov/ct2/show/record/NCT01753089?term=01753089?term&draw=2&rank=1 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |