Open Access Article
Open Access ArticleImpact of scaffoldin mechanostability on cellulosomal activity†
Albert
Galera-Prat
 *a,
Andrés M.
Vera
a,
Sarah
Moraïs
bc,
Yael
Vazana
b,
Edward A.
Bayer
*a,
Andrés M.
Vera
a,
Sarah
Moraïs
bc,
Yael
Vazana
b,
Edward A.
Bayer
 b and
Mariano
Carrión-Vázquez
b and
Mariano
Carrión-Vázquez
 *a
*a
aInstituto Cajal, Department of Molecular, Cellular and Developmental Neurobiology. IC-CSIC, Avenida Doctor Arce 37, 28002 Madrid, Spain. E-mail: mcarrion@cajal.csic.es; albert.galera@cajal.csic.es
bDepartment of Biomolecular Sciences, Weizmann Institute of Science, Rehovot, 76100, Israel
cDepartment of Life Sciences and the National Institute for Biotechnology in the Negev, Ben-Gurion University of the Negev, 8499000 Beer-Sheva, Israel
First published on 20th March 2020
Abstract
Lignocellulose is the most abundant renewable carbon source in the biosphere. However, the main bottleneck in its conversion to produce second generation biofuels is the saccharification step: the hydrolysis of lignocellulosic material into soluble fermentable sugars. Some anaerobic bacteria have developed an extracellular multi-enzyme complex called the cellulosome that efficiently degrades cellulosic substrates. Cellulosome complexes rely on enzyme-integrating scaffoldins that are large non-catalytic scaffolding proteins comprising several cohesin modules and additional functional modules that mediate the anchoring of the complex to the cell surface and the specific binding to its cellulosic substrate. It was proposed that mechanical forces may affect the cohesins positioned between the cell- and cellulose-anchoring points in the so-called connecting region. Consequently, the mechanical resistance of cohesins within the scaffoldin is of great importance, both to understand cellulosome function and as a parameter of industrial interest, to better mimic natural complexes through the use of the established designer cellulosome technology. Here we study how the mechanical stability of cohesins in a scaffoldin affects the enzymatic activity of a cellulosome. We found that when a cohesin of low mechanical stability is positioned in the connecting region of a scaffoldin, the activity of the resulting cellulosome is reduced as opposed to a cohesin of higher mechanical stability. This observation directly relates mechanical stability of the scaffoldin-borne cohesins to cellulosome activity and provides a rationale for the design of artificial cellulosomes for industrial applications, by incorporating mechanical stability as a new industrial parameter in the biotechnology toolbox.
Introduction
Cellulose is the most abundant biopolymer and carbon source in the biosphere. It is composed of glucose monomers that can be processed by specialized microorganisms to produce added-value chemicals such as biofuels. Cellulose is the major component of lignocellulosic biomass, which is found in enormous amounts around the world as a waste byproduct from agriculture and forestry or as an industrial or urban residue.1The use of lignocellulose to generate biofuels constitutes a scientific and technical challenge, since it is an insoluble, highly heterogeneous and recalcitrant material. It is generally recognized that the major bottleneck towards its utilization is the saccharification step, i.e. the breakdown of the polysaccharides to release the constituent soluble sugars.2–4 This process is carried out in nature by several microorganisms with specialized enzymatic machinery. In particular, some anaerobic bacteria produce and assemble extremely efficient high-molecular-weight cellulolytic complexes, called cellulosomes.5–8
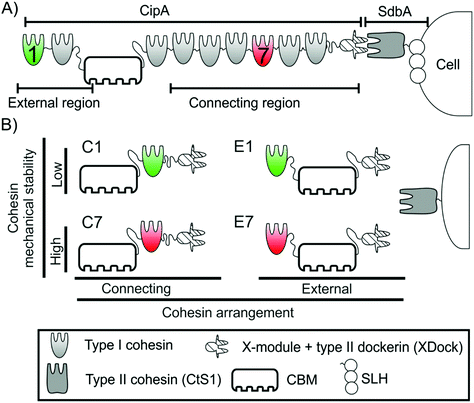
A unique cellulosomal feature is the scaffoldin subunit, a large and generally non-catalytic protein that anchors several complementary enzymes and directs their action towards the lignocellulosic substrate7 (Fig. 1A). This is achieved by the presence in scaffoldins of cohesin modules, that bind the dockerin modules of the enzymes with very high-affinity, as well as dockerin modules of an additional scaffoldin such as the anchoring scaffoldin, thus allowing hierarchical assembly of large complexes.7 In the most studied cellulosome producer, Clostridium thermocellum,9 the presence of a family 3a carbohydrate binding module (CBM) in the primary scaffoldin (i.e., a scaffoldin that directly binds the enzymes) allows the cellulosome to target its substrate7 while the SLH modules10 of anchoring scaffoldins provide a means for attachment to the cell surface.
The cohesin–dockerin interaction is of high affinity and specificity7,11,12 and, together with the modularity of cellulosomal components, provides the basis to establish the designer cellulosome technology, whereby the enzymatic composition can be defined and controlled.13–17 This technology also provides a scientific tool for determining the origin of the high catalytic activity usually observed in these complexes. This has been attributed both to the high activity of some of its enzymes as well as to their incorporation into the scaffoldin that promotes synergistic activity, resulting from proximity of complementary enzymes and targeting to the substrate through the CBM.14 Additionally, the anchoring of the cell to the substrate through the scaffoldin, which allows the formation of a cellulose-enzyme-microbe series, can further promote the activity of the system.18
It has been proposed that in nature the cellulosome can be exposed to mechanical stress.19–21 As in many adhesion systems,22 the relative movement of the cell and its substrate could stretch the cohesin modules placed in the region of the scaffoldin between the two anchoring points, i.e., cellulose to cellulosome and cellulosome to the cell surface. Since the application of mechanical force on a protein increases its unfolding probability,23 this may lead to forced unfolding of cohesins and subsequent dockerin (and enzyme) release. This scenario would negatively affect the overall activity of the cellulosome, since the synergy provided by scaffoldin binding would be lost. Thus, it was proposed that the mechanical stability of cohesins, i.e., the resistance to get unfolded by mechanical force, might influence cellulosome activity. It is important to note that this would only affect the cohesins located in the connecting region, i.e. those placed between the cell and cellulose anchoring points, but not the cohesins located in the external region (Fig. 1A).
The mechanical properties of several cohesins of the primary scaffoldin of C. thermocellum have been already characterized.19,20 These studies showed that cohesins located in the connecting region of primary scaffoldins, show high mechanical stability, while cohesins in the external region have much lower mechanical stability. Furthermore, the mechanical properties of these modules were not affected by the linker sequences that connect the cohesins, the presence of multiple cohesins in the scaffoldin nor the interaction with the dockerin (Fig. S1†).20
These results are in agreement with the mechanical hypothesis of the cellulosome19,20 but do not provide direct evidence regarding the effect of mechanical stability on the enzymatic activity of the system. Here, we directly address the relationship between the mechanical stability of cohesins and the cellulose-degrading activity of the cellulosome. To this end, we have designed four different monovalent cellulosomes (i.e., bearing single cohesins) with cohesins of known mechanical stability and studied their cellulolytic activity when bound to polystyrene microparticles (to mimic cell surface attachment) compared to that of free monovalent cellulosomes in solution.
Results
Engineering monovalent cellulosomes with cohesins of divergent mechanical stabilities
In order to study the effect of the mechanical stability of the scaffoldin-borne cohesins on the enzymatic activity of the resulting cellulosomes, we designed four monovalent cellulosomes inspired by the organization of the native cellulosome of C. thermocellum.These cellulosomes were selected according to the following two criteria: (i) cohesins of known mechanical stability were incorporated into a controlled position on a monovalent scaffoldin and (ii) the resultant scaffoldin should simultaneously bind both to the cell (herein represented experimentally by a polystyrene microparticle; see below) and to the substrate (cellulose).
Based on accumulated knowledge from previous works, we have chosen cohesins 1 and 7 from the C. thermocellum primary scaffoldin CipA. Cohesin 1 is located in the external region of the CipA scaffoldin. We recently measured its mechanical stability (at 400 nm s−1), which shows lower mechanical stability than cohesin 7 (124 ± 25 pN versus 480 ± 77 pN, respectively, mean ± sd), one of the cohesins placed in the connecting region of CipA scaffoldin, at the same pulling speed.19,20 The large difference in their mechanical stability make these cohesins excellent candidates for this comparative study about the influence of mechanical stability on enzymatic activity.
By incorporating the CBM and an X-module/type II dockerin modular pair (XDock) from C. thermocellum CipA, the resulting monovalent scaffoldins should bind to both microcrystalline cellulose and type II cohesins from this organism. The combination of these elements resulted in four different monovalent scaffoldins (Fig. 1B): two of them in a connecting arrangement, where the cohesin was placed between the CBM and the XDock and referred to as C1 (i.e., the cohesin 1 from CipA) and C7 (cohesin 7 of CipA), and another two where the respective cohesin was placed in the external region, referred to as E1 and E7 (Fig. 1). As described above, the portion of scaffoldins between the two anchoring points (i.e., its CBM and XDock) is expected to be subjected to mechanical stress: thus, in the C1 and C7 scaffoldins the cohesin would be affected by mechanical force unlike the case of E1 and E7 scaffoldins. Details on the cloning process and the sequences of the monovalent scaffoldins are shown in ESI.†
Assembly and functional activity of monovalent cellulosomes
Monovalent scaffoldins were recombinantly expressed and then analyzed using conventional biochemical techniques to check the functionality of their components (Fig. 2). Non-denaturing PAGE served to analyze if the XDock of the monovalent scaffoldins was capable of interacting with the type II cohesin from the SdbA scaffoldin protein (i.e., CtS1). Fig. 2B shows that the four monovalent scaffoldins exhibited an altered electrophoretic migration pattern when incubated in the presence of CtS1. As CtS1 concentrations increase, it was observed that the bands corresponding to the individual components disappeared at a molar ratio (monovalent scaffoldin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CtS1) of 1.1
CtS1) of 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for C1, C7 and E1 and 1.3
1 for C1, C7 and E1 and 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the E7 monovalent scaffoldin (data not shown). In all cases near the theoretical stoichiometric ratio of 1
1 for the E7 monovalent scaffoldin (data not shown). In all cases near the theoretical stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which indicates that the complexes were correctly formed.
1, which indicates that the complexes were correctly formed.
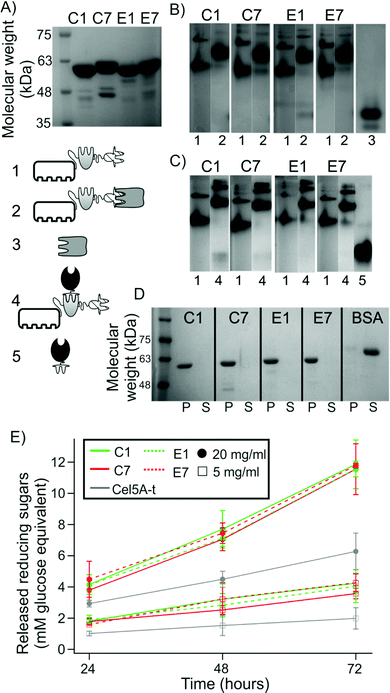 | ||
| Fig. 2 Monitoring the formation and functionality of the monovalent cellulosomes. (A) SDS-PAGE of purified monovalent scaffoldins. (B) Non-denaturing PAGE to assess the interaction of the scaffoldin-borne XDock with the type II CtS1-borne cohesin and (C) scaffoldin-borne type I cohesin with Cel5A-t. Lanes are labeled as: (1) monovalent scaffoldins, (2) monovalent scaffoldins with CtS1, (3) CtS1, (4) monovalent scaffoldin with Cel5A-t, (5) Cel5A-t alone. See pictograms. (D) Cellulose pull-down analysis by SDS-PAGE. P indicates the pelleted fraction while S refers to the supernatant. BSA was used as a standard negative control. (E) Activity of Cel5A-t alone or in the presence of equimolar concentrations of monovalent scaffoldin on 20 mg ml−1 or 5 mg ml−1 Avicel. Values are given as mean ± sd from nine independent measurements. Full gels with samples of B and C are shown in Fig. S2†). | ||
As can be observed, the four monovalent scaffoldins showed a double band pattern. According to the SDS-PAGE analysis (Fig. 2A) samples are above 95% purity, which indicates that the pattern observed in non-denaturing PAGE is not due to the presence of contaminant proteins. Furthermore, when interacting with CtS1, this double band pattern mostly disappears, which reinforces the interpretation that both bands correspond to scaffoldin. It has been observed that two CipA molecules can interact through the X-module and the following cohesin,24 the behavior observed here might be originated from partial dimerization of two monovalent scaffoldin molecules, although the possibility of misfolded XDock in monovalent scaffoldins originating two different populations cannot be ruled out.
A similar approach was used to study the capability of the scaffoldin-borne cohesins to interact with the type I dockerin module of an enzyme. Specifically, we studied the ability of the cohesins to bind to Cel5A-t, which consists of the Cel5A endocellulase from the non-cellulosome-producing thermophilic bacterium, Thermobifida fusca, fused to the dockerin module of the Xyn10Z xylanase from C. thermocellum, thereby converting the enzyme to the cellulosomal mode.25Fig. 2C shows that all four monovalent scaffoldins were capable of interacting with Cel5A-t. By varying the molar ratio of Cel5A-t to monovalent scaffoldin (Fig. S2B†), we found that the complex was formed at an enzyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) scaffoldin molar ratio of 0.9
scaffoldin molar ratio of 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, near the expected stoichiometric ratio (1
1, near the expected stoichiometric ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The actual experimentally determined value was used to calculate the amount of monovalent scaffoldin in subsequent reactions.
1). The actual experimentally determined value was used to calculate the amount of monovalent scaffoldin in subsequent reactions.
Finally, to ensure that the CBM was capable of interacting with microcrystalline cellulose, the different monovalent scaffoldins were incubated in the presence of Avicel, centrifuged to recover the fraction bound to the cellulose, and the released protein was analyzed by SDS-PAGE. Fig. 2D shows that the four scaffoldins appeared in the pellet fraction, while the control protein (bovine serum albumin, BSA), which is not able to bind cellulose, appears in the soluble fraction.
Since microcrystalline cellulose degradation assays are usually run for extended periods of time,26 it is important to study the stability of the monovalent cellulosome complexes under these conditions. To this end, the monovalent scaffoldins were bound to Cel5A-t or CtS1 in equimolar concentration and incubated at 50 °C for 72 h. Then, they were analyzed by SDS-PAGE and non-denaturing PAGE and compared to fresh samples (Fig. S4†). No significant loss or change in relative electrophoretic migration was observed for any of the complexes, which remained intact. Taken together, this set of results indicates that the monovalent scaffoldins behave as expected and are stable for the periods of time that are needed to study their activity.
Activity of free monovalent cellulosomes
We next studied the enzymatic activity of free monovalent cellulosomes on Avicel (Fig. 2E). For the two concentrations of substrate studied, we observed that the released soluble sugars linearly increased during the incubation time (72 h), indicating that activity was not saturated under these conditions. It was found that upon binding to each monovalent scaffoldin, Cel5A activity increased. This synergistic effect can be attributed to the targeting effect of the CBM present in the monovalent scaffoldin.25 The degree of synergy, measured as the ratio of activity of a monovalent cellulosome over the activity of free enzyme, was very similar for the four monovalent scaffoldins (for 20 mg ml−1 Avicel: 1.9 for C1 and E7 monovalent scaffoldins, and 1.8 for C7 and E1 monovalent scaffoldins; for 5 mg ml−1 substrate: 2.1 for C1 and E7, 2 for E1 and 1.8 for C7), which indicates that the different cohesins or arrangements used do not affect the activity of the free system. We also found that the degrees of synergy are reduced upon increasing substrate concentration. This effect is consistent with previously published results.14Binding of cellulosomes to microparticles to mimic bacterial-cell attachment
In order to mimic the attachment of cellulosomes to the bacterial cell and to apply mechanical forces to the monovalent designer cellulosomes, we used polystyrene microparticles capable of attaching them via the specific type II cohesin of anchoring scaffoldin CtS1, covalently coupled to the microparticle. This experimental design allowed us to apply mechanical stress to the elements of the monovalent scaffoldin placed between the two anchoring points.In order to closely mimic the size of the bacterial cell, we used polystyrene microparticles with an average size of 1.39 μm (see ESI†). These were amino-coated so that they could be easily functionalized using a water-soluble carbodiimide according to manufacturer instructions. The best conditions for covalent coupling of CtS1 were determined empirically. Under these conditions, 75% of the theoretical maximum binding capacity of the microparticles was reached.
Since this functionalization procedure is indiscriminate and binds exposed side-chain carboxyl groups (mainly aspartate and glutamate residues) of CtS1 to the polystyrene microparticles, we next analyzed the capacity of the functionalized, CtS1-coated microparticles to bind the type II XDock modular dyad. To this end, we used a GFP-XDock fusion protein that provides a fluorescent reporter protein capable of binding CtS1 (Fig. 3A). Incubation of this fluorescent probe with the CtS1-coated microparticles (Fig. 3B) resulted in a specific fluorescence signal, dependent on the concentration of both microparticle and GFP-XDock (though rapid saturation was observed when the GFP-XDock concentration was increased), compared to a non-binding fluorescent control protein (GFP-ssrA)27. The fluorescent probe allowed us to estimate that about 8 × 104 functional CtS1 molecules were available per particle, which is equivalent to 94 nmol of functional CtS1 per g of microparticles.
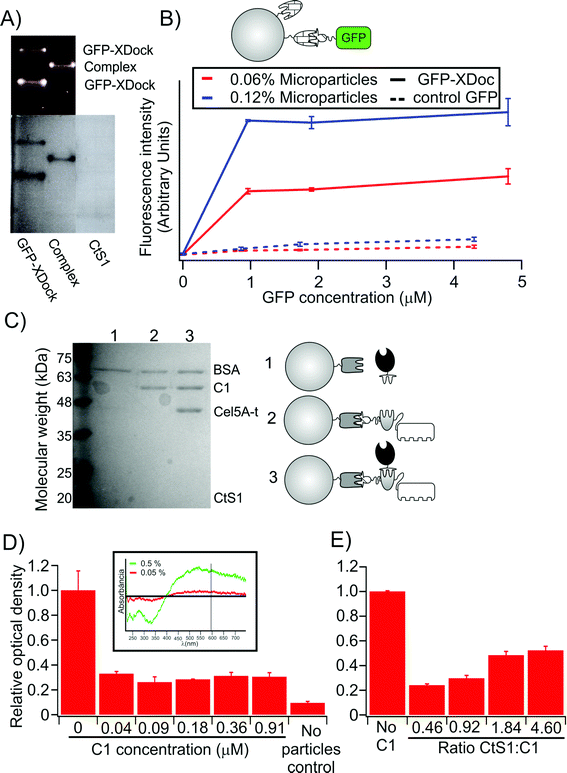 | ||
| Fig. 3 Control for the preparation and assembly of microparticles. (A) Non-denaturing PAGE of GFP-XDock and CtS1 seen under UV-light (top) and after Coomassie blue staining (bottom). (B) Quantification of functional CtS1 molecules on microparticles. The fluorescence signal, after incubating the type II cohesin-bearing CtS1-coated microparticles with GFP-XDock (continuous line), is compared to that obtained with non-interacting GFP-ssrA (dashed line), used as a control. (C) SDS-PAGE analysis of proteins bound to the CtS1-coated microparticles. As illustrated in the figure: lane 1, CtS1 bound covalently to the microparticles with Cel5A-t; lane 2, CtS1-coated microparticles with scaffoldin C1; lane 3, CtS1-coated microparticles with both scaffoldin C1 and Cel5A-t. BSA was added to all samples to quench unspecific interactions. The type I-bearing Cel5A binds to the CtS1-coated microparticles only in the presence of the type I-cohesins borne by the monovalent scaffoldin. (D) Cellulose pull-down of monovalent cellulosomes assembled on the CtS1-coated microparticles. The values are given as relative OD595 compared to the sample with no monovalent scaffoldin added. The inset shows that the OD595 of the microparticle suspension depends on the concentration of microparticles. (E) Cellulose pull-down of monovalent cellulosomes assembled on microparticles is reduced by addition of increasing free CtS1 concentration. Values in the figure are given as mean ± sd. The full gel corresponding to A is shown in Fig. S3.† | ||
Then, we analyzed the formation of monovalent cellulosome complexes on the functionalized microparticles. Monovalent scaffoldin C1 and pre-formed cellulosome C1 (i.e., dockerin-bearing enzyme bound to the scaffoldin) were incubated with the CtS1-coated microparticles. The microparticles were recovered by centrifugation, washed and analyzed by SDS-PAGE (Fig. 3C). In order to avoid unspecific interactions, BSA was added as a blocking agent at high concentration. As expected for correct assembly of the complex, we found that the functionalized microparticles were able to retain the Cel5A-t enzyme only when the monovalent scaffoldin was present.
Lastly, we analyzed the capacity of the monovalent cellulosomes assembled on the functionalized microparticles to bind cellulose (Fig. 3D). Monovalent scaffoldins were thus assembled onto the CtS1-bound microparticles, mixed with a suspension containing microcrystalline cellulose and allowed to settle for 10 min. The optical density at 595 nm of the supernatant fluids was measured, since the turbidity values are proportional to the concentration of microparticles suspended. The presence of monovalent scaffoldins clearly promotes sedimentation of the microparticles, indicating that their assembly onto the functionalized microparticle enabled binding to the cellulose substrate. Furthermore, this effect was reduced when excess free CtS1 was added to the sample (Fig. 3E), which effectively reduced the probability of monovalent scaffoldin to bind the microparticle.
Taken together, these results demonstrate that monovalent cellulosomes were correctly assembled onto the functionalized microparticles.
Cellulolytic activity is reduced under high mechanical stress upon using a less robust cohesin in the connecting region
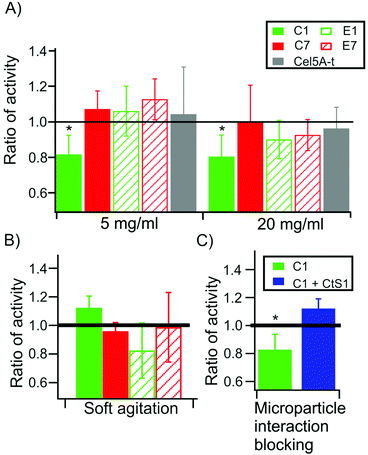
Then, the enzymatic activities of the monovalent cellulosomes assembled onto the functionalized microparticles were analyzed using magnetic stirring under high agitation conditions, in order to ensure that a high mechanical stress was introduced in the system. The results (Fig. 4) are presented as the ratio of activity of each type of monovalent cellulosome bound to the microparticles compared to the activity when they are free in solution. This ratio of activity allows normalization of small differences that may arise between different monovalent scaffoldins and provides a measure of the degree in which binding to the microparticle affects their activity. Thus, this value can be interpreted as the degree of synergy originated from the binding of the monovalent cellulosomes to the microparticles.Fig. 4A reveals that scaffoldin C1 presents a ratio of activity significantly below 1 (ratio of activity = 0.83 ± 0.12, p = 0.003 at 5 mg ml−1 substrate and 0.79 ± 0.11, p = 0.004 at 20 mg ml−1 Avicel). This indicates that, upon binding, the activity of this monovalent cellulosome, where a cohesin of low mechanical stability is located in the connecting region, was reduced. For the other monovalent cellulosomes (C7, E1 and E7) the ratio is indistinguishable from 1 at the two substrate concentrations assayed, indicating that there is no effect on their activity.
Remarkably, this effect is only apparent when we use high agitation during the incubation. When the experiments were repeated using much softer agitation, so that the mechanical stress on the monovalent cellulosomes was reduced, the ratio of activity for all monovalent cellulosomes was indistinguishable from 1 (Fig. 4B). Thus, at low agitation, the presence of the microparticle does not affect the activity of the monovalent cellulosomes, even for C1.
As an additional control, we performed activity assays in the presence of the type II cohesin of CtS1 free in solution (Fig. 4C). This competing protein was added in molar excess to the monovalent cellulosomes, in order to displace the binding equilibrium from the CtS1-coated microparticles towards the free protein. Monovalent cellulosomes were therefore in solution albeit in the presence of the microparticles. Using this approach, the ratio of activity was restored to the level of the cellulosomes in solution, supporting the conclusion that the observed reduction in activity originates from preventing the binding of the monovalent cellulosome to the functionalized microparticle. Furthermore, this control also rules out the possibility that CtS1 binding to monovalent scaffoldin has an effect on the activity of the cellulosome.
Discussion
Our working hypothesis regarding the higher mechanostability of connecting cohesins applies to those cellulosomes that are bound to both the substrate and to a cell, as is the case of C. thermocellum. To study the relationship between cellulolytic activity of the cellulosomes and mechanical properties of the scaffoldins, we have developed a system that incorporates all the necessary requirements for such analysis: (1) controlled mechanical stability, (2) control of cohesin position and (3) capability to bind to both cellulose and to a particle that emulates the bacterial cell. All monovalent scaffoldins used in this study included a single cohesin. Thus, it can be expected that synergistic activity is provided by the targeting effect of the CBM, which facilitates interpretation of the results and is of general applicability.We used polystyrene microparticles to emulate the effect of the presence of the cell while maintaining the geometry of force application (see ESI†). The microparticle size was selected in order to closely resemble the volume and surface of C. thermocellum cells (see ESI†). Thus, the particles are expected to reproduce the effects of the presence of an inert bacterial cell bound to the monovalent cellulosomes.
The attachment of a cellulosome onto the microparticles itself is of interest, owing to its potential industrial application, since it might allow exploitation of the properties of the microparticles to facilitate recycling of the cellulosome.28 Covalent binding of the type II cohesin-bearing CtS1 to the microparticles for subsequent binding of monovalent scaffoldin is also advantageous for the objectives of this study. First, this allows control of the geometry of the interaction, ensuring that the cohesin will be placed in either the connecting or the external region as desired. Significantly, this approach is based on the same cohesin–dockerin interactions that dictate cellulosome formation, thus avoiding alternative non-covalent29 or stronger covalent interactions that may influence the results.
When comparing the activity of monovalent cellulosomes in the presence of microparticles with their activity free in solution, only C1 showed decreased activity. This decrease in activity was consistent at shorter times (Fig. S5†) and did not reflect differences in sequence or composition, since this effect was not observed for the other monovalent scaffoldins. Furthermore, upon reducing mechanical stress using softer agitation, the effect disappeared, confirming its connection to the mechanical properties of the scaffoldin-borne cohesin.
In our activity experiments, monovalent cellulosomes are added in excess compared to the available CtS1 binding sites on the microparticles. Therefore, a reduction in the concentration of microparticles led to a reduction in the ratio of bound-versus-free activity displayed by the C1 cellulosome. Furthermore, the presence in excess of free CtS1 competed with the immobilized CtS1 for interaction with monovalent cellulosomes. This resulted in an increase of the population of free monovalent cellulosomes, so that the ratio of activity of C1 is less affected, thus confirming that the effect is related to the binding of the complex with the microparticle.
Taken together, these results indicate that the role of the reported high mechanical stability of primary scaffoldin-borne type I cohesins19,20 is to protect these modules from mechanical unfolding during the enzymatic activity of the complex.
The measured value of the ratio of activity for the C1 cellulosome represents a lower boundary for this parameter. This is due to the fact that the monovalent cellulosome is found in excess compared to the number of available binding sites on the microparticles. This situation could not be overcome, since relatively high concentrations of enzyme were needed to obtain a reasonable level of activity; notably, the immobilized enzyme has to work on an insoluble substrate (cellulose), thus steric considerations are evident, and the activity is consequently not usually very high. Indeed, the number of available binding sites on the microparticles was relatively low, making infeasible the amount of microparticles needed to bind all the monovalent cellulosomes. Taking into account these drawbacks, the actual ratio of activity for C1 may be even lower than the observed values presented in this work.
It is difficult to estimate the magnitude of forces that may be acting on cellulosomes in their natural environment, since the whole system is highly complex. Nevertheless, in the digestive tract of ruminants, in the soil or in other ecosystems inhabited by cellulosome-producing bacteria, relatively high forces may arise from hydrodynamic flows generated from gradients.30 In fact, cellulosome samples where a unidirectional flow was applied, showed partially disrupted scaffoldins, from which enzymes were released.31
Although in this work we only considered the mechanical stability of cohesins, that of the anchoring elements may also play important roles. The mechanical properties of XDock and CtS1 interaction have not been experimentally studied, although other cohesin–dockerin interactions show relatively high mechanical stability.32,33 Indeed, for the Ruminococcus flavefaciens CttA dockerin and ScaE cohesin, that are responsible of anchoring the cell to the cellulosic substrate, unbinding forces higher than those reported for the mechanical stability of cohesins have been reported, at similar loading rates.33 Furthermore, a study showed that force propagates through that cohesin–dockerin interaction in a way that minimizes the load on the interaction surface.34 In a theoretical study, the mechanical stability of cohesin–dockerin interaction was found to be highly dependent on the pulling geometry.35 When pulled using a geometry in which force may act on the system, these showed higher mechanical stability and more defined unfolding patterns. All this may suggest that cohesin–dockerin interaction has been adapted to resist forces.
The mechanical stability of the CBM–cellulose interaction has been addressed in several studies.36,37 These studies report relatively low unbinding forces, which would render this interaction the least stable of the system. Nevertheless, some aspects have to be taken into consideration. First, CBM unbinding and cohesin unfolding forces can only be directly compared at the same loading rate, that should be that reflected by the system. Second, even in the case where CBM unbinding occurs at lower forces than cohesin unfolding, both processes would compete. Upon CBM unbinding, since several binding sites are available on the surface of the lignocellulosic material, it is reasonable to expect that the CBM could re-attach shortly after unbinding. On the other hand, cohesin unfolding would result in a less effective cellulosome, especially considering that mechanically unfolded cohesins do not refold very efficiently. Therefore, CBM detachment may be more likely to occur but the system would be rapidly restored, whereas cohesin unfolding would lead to decreased activity.
We have argued that when cellulosomes are anchored to two different objects simultaneously, mechanical forces may be applied to the region placed between the two anchoring points. At a first glance, considering the anchoring points to be the CBM in the scaffoldin and its attachment system to the cell surface, may appear too simplistic since several cellulosome-incorporated enzymes are multimodular and include their own CBM.38,39 In principle, it should be noted that these CBMs would restrict the pathway through which force might propagate in the scaffoldin rendering the mechanical stability of cohesins less important for cellulosome activity.
Nevertheless, it has been observed that these CBMs are accessory modules that allow each particular enzyme to target more efficiently to its preferred site on the substrate but do not provide a strong attachment.39 This role appears to be restricted to the scaffoldin CBM, and as a result, we expect that the observed behavior could be extrapolated to whole cellulosomes.
Our data show a clear relationship between the activity of the cellulosome and the mechanical stability of its cohesins, which highlights the importance of mechanical stability as a parameter of industrial interest in biotechnology. These results allow us to provide a couple of basic guidelines for the design of more efficient designer cellulosomes according to their mechanical properties: (1) for cellulosomes with two attachment points, the cohesins between the anchoring points should be of high mechanical stability in order to avoid loss of activity; (2) for other types of cellulosomes, the mechanical stability of cohesins is not critical. Finally, it is interesting to note that these same rules appear to have shaped the evolution of the variety of architectures present in natural cellulosomes.
Methods
Protein engineering
The methods used to generate the desired constructions were based on standard procedures40 as applied for work on cellulosomal components.26,41 The elements of the monovalent scaffoldins were obtained from the C. thermocellum CipA scaffoldin. The four monovalent scaffoldins contained a CBM, an XDock and a cohesin in the following arrangement: pET28-CBM-CtA1-XDock (C1), pET28-CBM-CtA7-XDock (C7), pET28-CtA1-CBM-XDock (E1) and pET28-CtA7-CBM-XDock (E7). All monovalent scaffoldins carried a hexa-His tag at the N-terminus. Other clones used and protein purification methods are described in the ESI.†Monovalent cellulosome analysis
To assess cohesin–dockerin complex formation, samples were incubated at 37 °C for 1 h in activity buffer (50 mM Tris, 300 mM NaCl, 1 mM CaCl2 at pH 7.4) and then analyzed by non-denaturing PAGE.26 When GFP-XDock was used, a UV picture of the gel was taken (using GelPrinter Plus, TDI) before staining with Coomassie Brilliant Blue. The stability of the monovalent cellulosomes was assessed by comparing the migration pattern, both in non-denaturing and SDS-PAGE, of samples before and after being incubated for 72 h at 50 °C.CBM interaction with crystalline cellulose was studied as described previously.26 Bovine Serum Albumin (BSA, Sigma-Aldrich Inc., St Louis, MO) was used as a negative-control, non-binding protein.
Microparticle functionalization
Amino-coated polystyrene beads (Spherotech Inc., Lake Forest, IL) (5% w/v, 1.39 μm diameter) were recovered by centrifugation at 3000g for 15 min and washed twice in 50 mM 2-(N-morpholino)ethanesulfonic acid, pH 5 (MES buffer). Then the following coupling procedure was used: in 2 ml of 50 mM MES buffer, 0.2 ml of beads, 2 mg of CtS1 and 20 mg of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, Sigma Aldrich) were added. Samples were vortexed for 2 min, incubated at room temperature and further vortexed every 15 min for 2 h. Beads were then centrifuged 15 min at 3000g and the pellet washed three times in activity buffer. Total bound protein was determined as the difference between the initially added protein and that remaining in the supernatant after the coupling with EDC, using the Bradford reagent (Biorad Laboratories, Inc., Hercules, CA).Quantification of accessible CtS1 on microparticles was carried out by using a GFP-XDock probe. Particles and protein were incubated in activity buffer at 37 °C for 1 h. Particles were then recovered by centrifugation at 3000g for 15 min, washed twice in the same buffer and transferred to a 96 well plate. Remaining fluorescence on beads was measured using a FLUOStar OPTIMA fluorimeter (BMG Labtech, Offenburg, Germany). GFP-ssrA (ESI†) was used as a negative control protein as it does not bind CtS1.
Monovalent cellulosome assembly on microparticles
Monovalent scaffoldins and/or Cel5A were incubated with the microparticles at 37 °C for 1 h in activity buffer. The beads were then centrifuged at 3000g for 15 min and washed twice in the same buffer. The pellet samples containing the microparticles were heated at 98 °C for 5 min in loading buffer (150 mM Tris [pH7.5], 10% glycerol, 1% SDS, 0.002% bromophenol blue, 5% beta-mercaptoethanol) and then loaded in an SDS-PAGE gel for analysis. Using this approach, only proteins non-covalently bound to the particles were resolved in the gel since the microparticles cannot enter the gel. BSA was added to all samples to block non-specific interactions with the beads. Cel5A alone (without monovalent scaffoldin) was used as a negative control.Functionalized particles (0.15%) with bound monovalent scaffoldins were incubated in the presence of Avicel (microcrystalline cellulose, Sigma-Aldrich Inc., St Louis, MO) at room temperature for 10 min. Supernatant fluids (100 μl) were then collected and diluted in 900 μl of activity buffer, and the turbidity (OD at 595 nm) was determined.
Substrate
Avicel (microcrystalline cellulose, Sigma-Aldrich Inc., St Louis, MO) was used, since it has been described that synergy of designer cellulosomes (assembly of enzymes into a CBM-bearing scaffoldin) is larger than in more accessible substrates, such as phosphoric acid-treated cellulose (PASC or amorphous cellulose) or soluble carboxymethyl cellulose.14,26 A single batch was used for all the experiments and samples were prepared immediately before starting the experiments following the same protocol.Activity assays
The activity of the monovalent cellulosomes on microcrystalline cellulose (Avicel) was studied by incubation of pre-assembled monovalent cellulosomes in the presence of Avicel at 50 °C for 72 h under magnetic agitation. A homemade magnetic stirrer with speed regulation was used. In each sample tube (2 ml) a wing magnetic bar (9 × 5.5 mm, VWR) was introduced. Stirring speed was modified until all magnets were actively moving, resulting in high agitation and good mixing of the Avicel in the samples, as assessed visually. Up to 60 tubes were incubated in each experiment. Assays at low agitation were performed in 1.5 ml tubes in an Innova 4430 (New Brünswick, Eppendorf, Germany) orbital shaker at 300 rpm.Each sample was prepared as follows: 0.5 μM enzyme was incubated in activity buffer with monovalent scaffoldin in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as experimentally. BSA was also added at 1 mg ml−1, and samples were incubated for 1 h at 37 °C. When necessary, CtS1-coated polystyrene beads were added to the corresponding samples at a concentration of 0.33% w/v and incubated 1 h at 37 °C. Finally, Avicel, at a final concentration of 5 or 20 mg ml−1, was added. The winged magnet was introduced in the tubes and samples were incubated at 50 °C for 72 h in an oven.
1 ratio as experimentally. BSA was also added at 1 mg ml−1, and samples were incubated for 1 h at 37 °C. When necessary, CtS1-coated polystyrene beads were added to the corresponding samples at a concentration of 0.33% w/v and incubated 1 h at 37 °C. Finally, Avicel, at a final concentration of 5 or 20 mg ml−1, was added. The winged magnet was introduced in the tubes and samples were incubated at 50 °C for 72 h in an oven.
Cellulase activity was monitored by measuring the released soluble sugars, using the 3,5-dinitrosalicylic acid (DNS) assay.26,41 To measure the outcome of the reactions, 200 μl of each sample were loaded in a 96-well plate to determine OD540 in a FLUOStar optima fluorimeter in the absorbance mode. The glucose equivalent concentration was calculated using a glucose standard curve.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the members of the M.C.-V. lab for critical reading of the manuscript. We also appreciate the technical assistance of Melina Shamshoum and scientific advice from Drs. Ilit Noach and Yoav Barak (Chemical Research Support, Weizmann Institute). This work was supported by a Seventh Framework Programme in Nanosciences, Nanotechnologies, Materials & New Production Technologies (7PM-NMP 2013–17, 604530-2, CellulosomePlus) and the ERA-IB-ERANET-2013–16 (EIB.12.022, FiberFuel) through the Spanish MINECO (PCIN-2013-011-C02-01). AGP acknowledges financial support from an FPU fellowship of the Spanish MECD.References
- E. A. Bayer, R. Lamed and M. E. Himmel, The potential of cellulases and cellulosomes for cellulosic waste management, Curr. Opin. Biotechnol, 2007, 18(3), 237–245 CrossRef CAS PubMed.
- L. R. Lynd, et al., Microbial cellulose utilization: fundamentals and biotechnology, Microbiol. Mol. Biol. Rev., 2002, 66(3), 506–577 CrossRef CAS PubMed.
- A. L. Demain, M. Newcomb and J. H. Wu, Cellulase, clostridia, and ethanol, Microbiol. Mol. Biol. Rev., 2005, 69(1), 124–154 CrossRef CAS PubMed.
- B. A. White, et al., Biomass utilization by gut microbiomes, Annu. Rev. Microbiol., 2014, 68, 279–296 CrossRef CAS PubMed.
- E. A. Bayer, et al., From cellulosomes to cellulosomics, Chem. Rec., 2008, 8(6), 364–377 CrossRef CAS PubMed.
- E. A. Bayer, Y. Shoham and R. Lamed, Lignocellulose-decomposing bacteria and their enzyme systems, in The Prokaryotes, Springer, 2013, p. 215–266 Search PubMed.
- E. A. Bayer, et al., The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides, Annu. Rev. Microbiol., 2004, 58, 521–554 CrossRef CAS PubMed.
- L. Artzi, E. A. Bayer and S. Morais, Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides, Nat. Rev. Microbiol., 2017, 15(2), 83–95 CrossRef CAS PubMed.
- R. Lamed, E. Setter and E. A. Bayer, Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum, J. Bacteriol., 1983, 156(2), 828–836 CrossRef CAS PubMed.
- E. Leibovitz and P. Beguin, A new type of cohesin domain that specifically binds the dockerin domain of the Clostridium thermocellum cellulosome-integrating protein CipA, J. Bacteriol., 1996, 178(11), 3077–3084 CrossRef CAS PubMed.
- M. Slutzki, et al., Indirect ELISA-based approach for comparative measurement of high-affinity cohesin-dockerin interactions, J. Mol. Recognit., 2012, 25(11), 616–622 CrossRef CAS PubMed.
- R. Haimovitz, et al., Cohesin-dockerin microarray: Diverse specificities between two complementary families of interacting protein modules, Proteomics, 2008, 8(5), 968–979 CrossRef CAS PubMed.
- H. P. Fierobe, et al., Design and production of active cellulosome chimeras. Selective incorporation of dockerin-containing enzymes into defined functional complexes, J. Biol. Chem., 2001, 276(24), 21257–21261 CrossRef CAS PubMed.
- H. P. Fierobe, et al., Degradation of cellulose substrates by cellulosome chimeras. Substrate targeting versus proximity of enzyme components, J. Biol. Chem., 2002, 277(51), 49621–49630 CrossRef CAS PubMed.
- S. Morais, et al., Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes, mBio, 2012, 3(6), pii: e00508-12 CrossRef PubMed.
- J. Stern, et al., Adaptor Scaffoldins: An Original Strategy for Extended Designer Cellulosomes, Inspired from Nature, mBio, 2016, 7(2), e00083 CrossRef CAS PubMed.
- L. Davidi, et al., Toward combined delignification and saccharification of wheat straw by a laccase-containing designer cellulosome, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(39), 10854–10859 CrossRef CAS.
- Y. Lu, Y. H. Zhang and L. R. Lynd, Enzyme-microbe synergy during cellulose hydrolysis by Clostridium thermocellum , Proc. Natl. Acad. Sci. U. S. A., 2006, 103(44), 16165–16169 CrossRef CAS PubMed.
- A. Valbuena, et al., On the remarkable mechanostability of scaffoldins and the mechanical clamp motif, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(33), 13791–13796 CrossRef CAS PubMed.
- A. Galera-Prat, et al., The cohesin module is a major determinant of cellulosome mechanical stability, J. Biol. Chem., 2018, 293(19), 7139–7147 CrossRef CAS PubMed.
- T. Verdorfer, et al., Combining in Vitro and in Silico Single-Molecule Force Spectroscopy to Characterize and Tune Cellulosomal Scaffoldin Mechanics, J. Am. Chem. Soc., 2017, 139(49), 17841–17852 CrossRef CAS PubMed.
- C. Bustamante, et al., Mechanical processes in biochemistry, Annu. Rev. Biochem., 2004, 73, 705–748 CrossRef CAS PubMed.
- A. Galera-Prat, et al., Understanding biology by stretching proteins: recent progress, Curr. Opin. Struct. Biol., 2010, 20(1), 63–69 CrossRef CAS PubMed.
- J. J. Adams, et al., Insights into higher-order organization of the cellulosome revealed by a dissect-and-build approach: crystal structure of interacting Clostridium thermocellum multimodular components, J. Mol. Biol., 2010, 396(4), 833–839 CrossRef CAS PubMed.
- J. Caspi, et al., Effect of linker length and dockerin position on conversion of a Thermobifida fusca endoglucanase to the cellulosomal mode, Appl. Environ. Microbiol., 2009, 75(23), 7335–7342 CrossRef CAS PubMed.
- Y. Vazana, et al., Designer cellulosomes for enhanced hydrolysis of cellulosic substrates, Methods Enzymol., 2012, 510, 429–452 CAS.
- N. Benaroudj and A. L. Goldberg, PAN, the proteasome-activating nucleotidase from archaebacteria, is a protein-unfolding molecular chaperone, Nat. Cell Biol., 2000, 2(11), 833–839 CrossRef CAS PubMed.
- J. Krauss, V. V. Zverlov and W. H. Schwarz, In vitro reconstitution of the complete Clostridium thermocellum cellulosome and synergistic activity on crystalline cellulose, Appl. Environ. Microbiol., 2012, 78(12), 4301–4307 CrossRef CAS PubMed.
- M. Conti, G. Falini and B. Samori, How Strong Is the Coordination Bond between a Histidine Tag and Ni - Nitrilotriacetate? An Experiment of Mechanochemistry on Single Molecules, Angew. Chem., Int. Ed., 2000, 39(1), 215–218 CrossRef CAS.
- Marcos, Bacteria in shear flow, Massachusetts Institute of Technology, 2010 Search PubMed.
- M. Madkour and F. Mayer, Structural organization of the intact bacterial cellulosome as revealed by electron microscopy, Cell Biol. Int., 2003, 27(10), 831–836 CrossRef CAS.
- S. W. Stahl, et al., Single-molecule dissection of the high-affinity cohesin-dockerin complex, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(50), 20431–20436 CrossRef CAS PubMed.
- C. Schoeler, et al., Ultrastable cellulosome-adhesion complex tightens under load, Nat. Commun., 2014, 5, 5635 CrossRef CAS.
- C. Schoeler, et al., Mapping Mechanical Force Propagation through Biomolecular Complexes, Nano Lett., 2015, 15(11), 7370–7376 CrossRef CAS PubMed.
- M. Wojciechowski, D. Thompson and M. Cieplak, Mechanostability of cohesin-dockerin complexes in a structure-based model: anisotropy and lack of universality in the force profiles, J. Chem. Phys., 2014, 141(24), 245103 CrossRef.
- J. R. King, C. M. Bowers and E. J. Toone, Specific binding at the cellulose binding module-cellulose interface observed by force spectroscopy, Langmuir, 2015, 31(11), 3431–3440 CrossRef CAS PubMed.
- M. Zhang, B. Wang and B. Xu, Measurements of single molecular affinity interactions between carbohydrate-binding modules and crystalline cellulose fibrils, Phys. Chem. Chem. Phys., 2013, 15(17), 6508–6515 RSC.
- B. Dassa, et al., Genome-wide analysis of acetivibrio cellulolyticus provides a blueprint of an elaborate cellulosome system, BMC Genomics, 2012, 13, 210 CrossRef CAS PubMed.
- E. Morag, et al., Dissociation of the cellulosome of Clostridium thermocellum under nondenaturing conditions, J. Biotechnol., 1996, 51(3), 235–242 CrossRef CAS.
- J. Sambrook and D. W. Russell, Molecular cloning: a laboratory manual, CSHL Press, 2001 Search PubMed.
- A. Kahn, E. A. Bayer and S. Morais, Advanced Cloning Tools for Construction of Designer Cellulosomes, Methods Mol. Biol., 2018, 1796, 135–151 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9bm02052g |
| This journal is © The Royal Society of Chemistry 2020 |